Abstract
Although bendamustine has been used to treat lymphoproliferative disorders for decades, it has only recently been approved for use in Canada. Thus, Canadian recommendations on the administration of bendamustine and the management of common adverse events (aes) are needed. This article highlights effective management and assessment strategies recommended by Canadian nurses and pharmacists for the most common aes arising from the use of bendamustine in patients with chronic lymphocytic leukemia and indolent non-Hodgkin lymphoma. Those strategies include administering bendamustine over 60 minutes instead of 30 minutes, administering pre-medications to control infusion-related reactions and nausea, hydrating patients to minimize fatigue, and using free-flowing saline at the closest port to prevent phlebitis.
Keywords: Bendamustine, adverse events, toxicities, chronic lymphocytic leukemia, indolent non-Hodgkin lymphoma, nhl, cll, inhl, fatigue, nausea
1. BACKGROUND
Bendamustine is a chemotherapeutic agent recently approved by Health Canada for patients with relapsed indolent non-Hodgkin lymphoma (inhl) that is not responding adequately to a rituximab regimen, and for patients with chronic lymphocytic leukemia (cll) who have received no prior treatment1. The approval by Health Canada in August 2012 comes after an earlier approval by the U.S. Food and Drug Administration in 20082, and after decades of clinical experience with the drug that began in the former German Democratic Republic3.
Bendamustine—first synthesized in 1963 in the German Democratic Republic—is one of a series of nitrogen compounds containing the benzimidazole ring structure that resembles the purine structure4. The attempt by Ozegowski and colleagues to synthesize this new agent was motivated not only by a need to overcome the high cost of alkylating agents available from the West, but also to reduce toxicity without sacrificing antitumour activity4,5. The result was a unique, potentially bifunctional agent comprising a 2-chloroethylamine alkylating group similar to that found in cyclophosphamide, chlorambucil, and melphalan; a benzimidazole ring similar to that seen in purine analogues such as fludarabine; and a butyric acid side chain similar to that seen in chlorambucil.
Although the details of bendamustine’s mechanism of action remain unclear, this agent differs from other alkylating agents in its more complex structure and the more extensive and lasting dna damage it causes6. With typical alkylating agents, dna damage leads to apoptosis only. However, with bendamustine, the cell undergoes apoptosis or a chaotic form of cell death called mitotic catastrophe, which is thought to be caused by bendamustine inflicting dna damage and simultaneously forcing the cell to continue with cell division despite the unrepaired damage. The result is a failure to complete mitosis because of the improper alignment and segregation of severely damaged chromosomes. Eventually the cell stalls in mitosis and dies. This mechanistic attribute of bendamustine might explain why bendamustine remains active against primary nhl cells that are refractory to cyclophosphamide and doxorubicin7: cells have more difficulty developing resistance to both mitotic catastrophe and apoptosis than to apoptosis only. Consistent with these in vitro observations, bendamustine also shows activity in clinical settings in which other active alkylating agents have lost efficacy6.
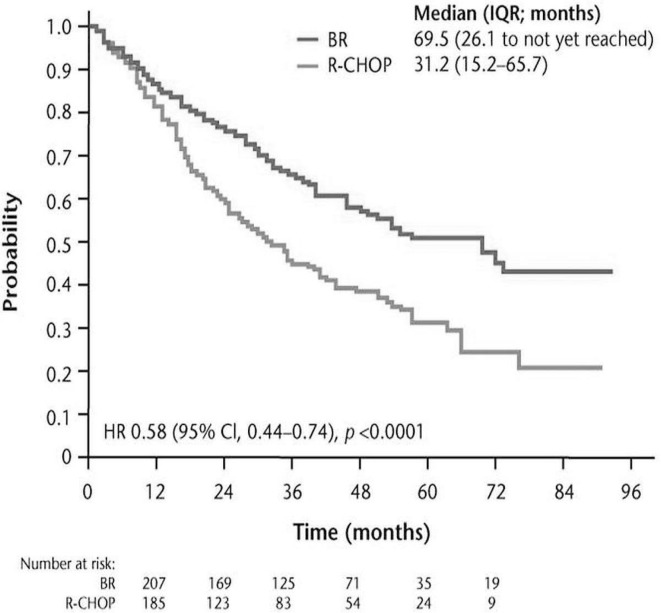
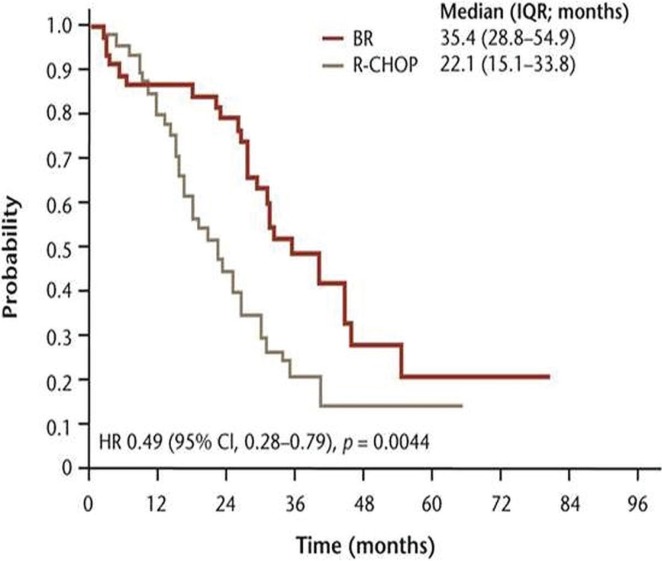
Although bendamustine was first used clinically in 1969 for multiple myeloma in the German Democratic Republic3, systematic studies of its action in lymphoproliferative disorders began only in the 1990s, after the fall of the Berlin Wall, which permitted the use of bendamustine by Western countries. Two pivotal clinical trials brought bendamustine to the forefront of chemotherapeutic therapies for inhl and cll. In 2003, Rummel et al. began a randomized phase iii study (stil-1) comparing bendamustine and rituximab (br) with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (r-chop) in patients with untreated inhl and mantle cell lymphoma (mcl)8. The much anticipated results of the trial were published in 2013 (Figures 1 and 2).
FIGURE 1.
Progression-free survival for all patients (adapted from Rummel et al., 20138). br = bendamustine, rituximab; r-chop = rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; iqr = interquartile range; hr = hazard ratio; ci = confidence interval.
FIGURE 2.
Progression-free survival by histologic subtype: mantle cell lymphoma (adapted from Rummel et al., 20138). br = bendamustine, rituximab; r-chop = rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; iqr = interquartile range; hr = hazard ratio; ci = confidence interval.
Although stil-1 was designed to show the non-inferiority of br compared with r-chop, and although overall survival was not significantly different between the groups, the trial clearly showed that br should be considered a preferred first-line treatment in this patient group because of a significantly improved rate of progression-free survival (pfs) and a significantly better toxicity profile with respect to hematologic toxicity, infections, peripheral neuropathy, and stomatitis. However, compared with patients receiving r-chop, those treated with br had a higher frequency of erythematous skin reactions (urticaria and rash: 16% vs. 9%; p = 0.024). With respect to hematologic toxicity, the authors noted a significant difference in the frequency of neutropenia in favour of the br arm, with an associated decrease in the use of hematopoietic growth factors. In addition, the br group showed a complete absence of alopecia (Table i), an observation that we have observed to be very important to patients in practice.
TABLE I.
Key findings of the Rummel et al.8,a study in the first-line treatment of indolent non-Hodgkin lymphoma
| Variable |
Chemotherapy regimen
|
p Value | |
|---|---|---|---|
| br (n=261) | r-chop (n=253) | ||
| Efficacy: pfs (months) | |||
| Median | 69.5 | 31.2 | <0.0001 |
| Range | 26.1–nrb | 15.2–65.7 | (hr: 0.58) |
| Toxicity (%) | |||
| Hematologic toxicity | 30 | 68 | <0.0001 |
| Peripheral neuropathy | 7 | 29 | <0.0001 |
| Stomatitis | 6 | 19 | <0.0001 |
| Alopecia | 0 | 100c | <0.0001 |
| Erythematous skin reactions | 16 | 9 | 0.024 |
| Infection episodes | 37 | 50 | 0.0025 |
Median follow-up: 45 months.
At the time of publication, more than 50% of patients were free of disease progression.
Of patients receiving 3 or more cycles of treatment.
br = bendamustine, rituximab; r-chop = rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; pfs = progression-free survival; nr = not yet reached; hr = hazard ratio.
Currently, the first-line treatment for many patients in Canada is rituximab, cyclophosphamide, vincristine, and prednisolone (r-cvp)10. However, an ongoing phase iii clinical trial called bright will help to provide clarity on the comparative effects of r-cvp and br (search for NCT00877006 at http://clinicaltrials.gov/). The primary objective of the present study was to compare the rates of complete response in patients with advanced inhl or mcl treated with br or with the standard regimens r-cvp or r-chop.
Consistent with the efficacy of br observed in patients with inhl and mcl in the first-line setting, the stil-2 study by Rummel et al. showed superior efficacy for the br regimen over rituximab plus fludarabine in the relapsed setting (pfs: 30 months vs. 11 months; hazard ratio: 0.50; p < 0.0001)9. Overall survival was not significantly different between the arms. Patients in both arms experienced similar adverse events (aes).
Based on the Rummel studies (stil-1 and stil-2), the pan-Canadian Oncology Drug Review Expert Review Committee recommended that bendamustine in combination with rituximab be funded as first-line therapy in patients with inhl and mcl, concluding that bendamustine has a net clinical benefit and is likely to be cost-effective in that setting10. The committee made the same funding recommendations for bendamustine when used in combination with rituximab for the treatment of relapsed or refractory inhl and mcl.
In addition to the superior efficacy and safety of br compared with r-chop in patients with inhl and mcl, work independent from the stil-1 study showed that this patient group experiences a higher quality of life when treated with br. Those data, which were published by Burke et al.11 in abstract form, showed that global health status, physical function, social and emotional function, fatigue, shortness of breath, and constipation were improved in patients treated with br compared with those treated with r-chop.
Bendamustine has also demonstrated effectiveness in patients with cll. In 2009, a randomized phase iii clinical trial by Knauf et al.12 compared efficacy and safety between bendamustine and chlorambucil in untreated patients with cll. That study was recently updated in 201213. Median pfs at a median follow-up of 54 months was significantly greater in patients treated with bendamustine than in patients treated with chlorambucil (21.2 months vs. 8.8 months; hazard ratio: 2.83; p < 0.0001). Overall survival was not significantly different in the two arms. Compared with patients receiving chlorambucil, those treated with bendamustine experienced a higher frequency of hematologic toxicities (40% vs. 19%) and more frequently reported gastrointestinal complaints (nausea, vomiting, diarrhea). Despite the higher frequency of hematologic toxicity observed in the bendamustine arm, the rate of grades 3 and 4 infections remained relatively low with both bendamustine and chlorambucil (8% vs. 3%)7. This study by Knauf et al. became the basis for approval by the U.S. Food and Drug Administration, and later Health Canada, of bendamustine for untreated cll.
Although significant progress has been made in understanding the role of bendamustine compared with chlorambucil in untreated cll, the role of bendamustine compared with fludarabine in the same patient population remains unknown. In addition, evidence about the efficacy and safety of bendamustine in the relapsed or refractory setting in cll is limited, which led the pan-Canadian Oncology Drug Review Expert Review Committee not to recommend bendamustine in that setting.
2. ADMINISTERING BENDAMUSTINE
2.1. Preparation
Bendamustine is supplied as a lyophilized powder in 25 mg or 100 mg single-use vials (bendamustine plus mannitol)1. Bendamustine, which is for injection only, should be reconstituted with sterile water as close to administration time as possible because of its relative instability once dissolved. Within 5 minutes, the result should be a clear, colourless to pale-yellow solution; if any particulates are observed after that time, the vial should be discarded. Within 30 minutes, the bendamustine solution should be diluted with 500 mL 0.9% saline (or 2.5% dextrose and 0.45% sodium chloride, usp), after which the drug will be stable for 3 hours at room temperature or 24 hours refrigerated1.
2.2. Dose
When bendamustine is given as monotherapy for patients with relapsed inhl, the product monograph recommends an intravenous dose of 120 mg/m2 administered over 60 minutes on days 1 and 2 of a 21-day cycle, for up to 8 cycles1,14. In patients with untreated cll, the product monograph recommends an intravenous dose of 100 mg/m2 administered over 30 minutes on days 1 and 2 of a 28-day cycle, for up to 6 cycles. Although the recommendation to administer bendamustine in inhl over 21 days is based on studies that used that cycle length, many sites administer bendamustine over a 28-day cycle instead. Table ii sets out the recommended doses of bendamustine when used as monotherapy or in combination with rituximab for cll and inhl14,15.
TABLE II.
Dose recommendations for bendamustine therapya
| Indication | Dose [mg/m2 (on days 1 and 2)] |
|---|---|
| Chronic lymphocytic leukemia | |
| Initial therapy | |
| Single agent | 100 |
| With rituximabb | 90 |
| Relapsed or refractory | |
| Single agent | 70 |
| With rituximabb | 70 |
| Follicular or low-grade nhl | |
| Initial therapy | |
| With rituximabc | 90 |
| Relapsed or refractory | |
| Single agent | 120 |
| Relapsed or refractory | |
| With rituximab | 90 |
2.3. Administration
Bendamustine is considered to be an irritant, but not a vesicant (Cephalon. Data on file, 2011). As with all cytotoxic agents, nurses and pharmacists have to be aware of signs of possible extravasation (pain, redness), and irritation of the affected area should be managed following institutional guidelines1.
Bendamustine is currently recommended at an infusion time of 60 minutes in patients with inhl and 30 minutes in patients with cll. However, we recommend that the infusion time be standardized at 60 minutes in both patient groups. Our experience with bendamustine indicates that a reduced infusion time of 30 minutes seems to increase the associated infusion-related reactions (irrs), especially delayed irrs occurring 4–6 hours after infusion. Recent work by Owen et al. is consistent with that experience, showing a higher probability of nausea occurring at peak plasma concentration of the drug16. Short bendamustine infusions (30 minutes) might potentially be increasing the peak plasma concentration, which would explain those observations.
3. AEs AND THEIR MANAGEMENT
3.1. IRRs
The frequencies of all grades of irr occurring with the use of bendamustine have been reported in clinical trials as fever, 24%–34% (2% grade 3 or 4); chills, 1%–14% (0%–1% grade 3 or 4); pruritus, 6% (0% grade 3 or 4); and rash 5%–16% (1%–3% grade 3 or 4)17. More severe irrs and hypersensitivity reactions, including anaphylaxis, are not common, but nurses should always monitor for symptoms of an anaphylactic reaction (facial swelling and breathing difficulties). Although irrs can occur during any treatment cycle, they are more frequent after cycle 2. They can be effectively managed by prophylactic treatment, careful monitoring of symptoms, and patient education18,19. Because bendamustine is administered over a 2-day period, management with acetaminophen and diphenhydramine before each day’s infusion is recommended (Table iii). Patients should be made aware of the possibility that fever, rash, and chills could occur a few hours after infusion, and they should be given instructions to take acetaminophen or diphenhydramine that evening as required. If fever, rash, and chills develop on day 1, steroids should be administered on day 2, with treatment continued at the discretion of the physician.
TABLE III.
Management and assessment of key adverse events
| Event | Management (suggested treatment options) | Assessment |
|---|---|---|
| Infusion-related reactions (chills, fever, rash) | Premedication: acetaminophen, diphenhydramine, steroids—for example, methylprednisolone, prednisone for rash Patient education about the possible reactions |
During infusion or 4–6 hours after treatment Vital signs, monitor patient Document |
| Nausea and vomiting | Prophylactic 5-HT3 antagonist (for example, ondansetron), before and after infusion Rescue antiemetic—for example, prochlorperazine as needed Hydration Add dexamethasone or neurokinin 1 antagonists—for example, oral aprepitant for severe vomiting Patient education (encourage oral hydration) |
Frequency of nausea or vomiting Are prophylactic medications working? Oral intake in 24 hours Assess for dehydration May need to notify physician Prescriptions for home use Document |
| Fatigue | Intravenous pre-infusion of 250 mL normal saline, followed by intravenous normal saline 250–500 mL given concurrently with bendamustine infusion to help with fatigue and nausea Patient education (maintain adequate oral hydration), and management of chemotherapy-related fatigue (for more information visit http://youtu.be/YTFPMYGe86s) |
Ensure that intravenous saline is given pre-infusion Assess fatigue Document |
| Phlebitis | Free-flow intravenous normal saline 250–500 mL at port closest to infusion site, given concurrently during bendamustine infusion Start a new peripheral line for each infusion on days 1 and 2 Normal saline given post infusion for fatigue also clears any residual drug left in the tubing and helps to prevent phlebitis Advocate for consideration of central venous access devices per best practice guidelines from the Registered Nurses’ Association of Ontario |
Thorough assessment of veins Assess for pain, swelling, redness Day 2: assess day 1 intravenous site Document |
3.2. Hematologic AEs
The most common hematologic toxicities with bendamustine in cll and inhl are neutropenia, thrombocytopenia, and anemia (Table iv)1,8,12. Leukocytes (neutrophils and lymphocytes) and platelets typically reach nadir 14–20 days after bendamustine infusion, with recovery expected within 3–5 weeks19. In addition, the frequency of neutropenia tends to increase after cycle 3 and is generally higher in patients more than 65 years of age. Nurses and pharmacists should counsel patients on the anticipated effects of low blood counts and the signs and symptoms of possible infections near the nadir, especially after cycle 3 of treatment. In the event of grade 4 neutropenia [absolute neutrophil count (anc) < 0.5×109/L] or grade 4 thrombocytopenia (anc < 25×109/L), treatment delay until count recovery to grade 1 or 2 (platelets ≥ 1×109/L, platelets ≥ 50×109/L) is recommended.
TABLE IV.
Common hematologic adverse events associated with bendamustine monotherapy in first-line chronic lymphocytic leukemia (cll) and rituximab-refractory indolent B-cell non-Hodgkin lymphoma (inhl)a
3.3. Nonhematologic AEs
The most frequent nonhematologic aes associated with bendamustine are fatigue, nausea, vomiting, and diarrhea1. Table v presents the frequencies of nonhematologic aes.
TABLE V.
Common nonhematologic adverse events associated with bendamustine monotherapy in first-line chronic lymphocytic leukemia (cll) and rituximab-refractory indolent B-cell non-Hodgkin lymphoma (inhl)a
| Event | Occurrence (%) during therapy for | |||
|---|---|---|---|---|
| inhl | cll | |||
|
|
|
|||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Fatigue | 64 | 14 | 9 | 1 |
| Nausea | 77 | 4 | 19 | <1 |
| Vomiting | 40 | 2 | 16 | 1 |
| Diarrhea | 42 | 5 | 10 | 1 |
| Rash | 15 | 1 | 9 | 2 |
| Chills | 14 | 0 | 6 | 0 |
| Dehydration | 15 | 6 | ns | ns |
Adapted from Lundbeck Canada1.
ns = not stated in product monograph.
3.3.1. Fatigue
Fatigue is the second most common ae observed with bendamustine treatment in patients with inhl (64%); it is less frequent in patients with cll (9%)1. Based on educational, general, nonpharmacologic, and pharmacologic approaches, the U.S. National Comprehensive Cancer Network has published algorithms on managing cancer-induced fatigue21. Other education tools to help manage patients with fatigue and other side effects are also available22–25. An important factor that may exacerbate fatigue is dehydration. Nurses should educate patients about the need to increase their fluid intake before and after bendamustine infusions. To maintain adequate hydration, an additional step of pre-infusion with 250 mL normal saline (0.9% NaCl), followed by infusion with 250–500 mL normal saline given concurrently with the bendamustine infusion, can be considered. Experience has shown that this additional step of fluid delivery lessens nausea, improves fatigue management, and helps to prevent phlebitis. In addition, nurses should consider calling patients at home on day 3 after treatment to follow up with those at risk for continuing irrs.
3.3.2. Nausea and Vomiting
The Multinational Association of Supportive Care in Cancer classifies bendamustine as moderately emetogenic (30%–90% frequency) and has published an associated guideline on the management of chemotherapy-related nausea and vomiting26. Depending on the emetic risk category (determined by the physician case by case), patients treated with bendamustine should be given an anti-emetic such as a 5-HT3 antagonist (for example, ondansetron) or dexamethasone before infusion (Table iii). In patients at higher emetic risk, dexamethasone can be given in combination with 5-HT3 antagonists. A prescription for a rescue anti-emetic such as prochlorperazine can also be given. Constipation, diarrhea, and headaches are potential aes associated with 5-HT3 antagonists. Consequently, nurses and pharmacists should educate patients on those anticipated aes, which are typically observed 1–2 days after start of the 5-HT3 antagonist. With respect to constipation, adequate hydration and stool softeners such as docusate are effective. Laxatives such as sennosides can be added if needed.
3.4. Other AEs
3.4.1. Phlebitis
Phlebitis is inflammation of a vein, commonly occurring at the site of an inserted catheter27. Early signs include pain, redness, and swelling at the catheter insertion site. If not managed immediately, thrombosis may develop, resulting in a palpable venous cord and fever at later stages.
Some effective options to consider in preventing phlebitis are the insertion of a separate venous inter-catheter on days 1 and 2, alternating arms if patients are unable to tolerate an intravenous line in the same arm for 2 days, and the use of 250–500 mL free-flow saline into an intravenous port close to the drug infusion site. A post-treatment flush with 10–20 mL normal saline into the port closest to the cannula can also help to reduce the risk of phlebitis by clearing the cannula of any residual bendamustine. In addition, nurses should assess patients for central venous access devices (Table iii)28.
4. DOSE REDUCTIONS AND DELAYS
Patients who develop severe toxicities should have their next bendamustine dose delayed or reduced, or both1. In the case of a grade 4 hematologic toxicity or a grade 3 or greater nonhematologic toxicity, bendamustine administration should be delayed until the nonhematologic toxicity recovers to grade 1 or lower, or until blood counts improve (anc ≥ 1×109/L, platelets ≥ 75×109/L). Table vi sets out the bendamustine dose reductions recommended in cll and inhl. Nurses should always monitor patients for signs of intolerance to treatment and remind physicians of the option to reduce the dose.
TABLE VI.
Dose reductions for bendamustine therapya
| Disease status | Dose reduction [mg/m2 (days 1 and 2)] |
|---|---|
| Chronic lymphocytic leukemia | |
| First-line | |
| Single agent | 100→70 |
| Rituximab combination | 90→60 |
| Relapsed or refractory | |
| Single agent | 100→70 |
| Rituximab combination | 70→50b |
| Indolent B-cell nhl | |
| Rituximab combination | 90→60 |
| Single agent | 120→90→60 |
5. OTHER CONSIDERATIONS
5.1. Drug Interactions
Bendamustine is metabolized primarily by hydrolysis to form inactive metabolites16. A secondary metabolic route through the enzyme cytochrome P450 1A2 (CYP1A2) produces two active but minor metabolites1. Compared with the concentration of the parent compound, plasma concentrations of the active metabolites are very low. Studies examining the effects of inhibitors and inducers of CYP1A2 on the pharmacokinetics of bendamustine have been limited. One recent study found no evidence of a change in systemic exposure to bendamustine in the presence of inducers or inhibitors of CYP1A229. However, because those interactions remain understudied, nurses and pharmacists should educate patients on the possibility that smoking and medications such as ciprofloxacin may affect the efficacy of bendamustine.
5.2. Hepatic and Renal Impairment
The product monograph recommends caution when using bendamustine in patients with a creatinine clearance of 40–80 mL/min and does not recommend the use of bendamustine in patients with a creatinine clearance of less than 40 mL/min1. Bendamustine should be used with caution in patients with mild hepatic impairment: total bilirubin greater than the upper limit of normal (uln) – 1.5 × uln; or aspartate aminotransferase, alanine transaminase, or alkaline phosphatase greater than uln – (2.5 × uln). Bendamustine should not be used in patients with moderate or severe hepatic impairment.
Studies examining the effects of renal and hepatic impairment on bendamustine pharmacokinetics are limited. However, recent work suggests that the pharmacokinetics of bendamustine are not affected by renal impairment. That observation is consistent with urinary excretion being a relatively minor pathway of elimination for bendamustine1. In a study investigating how bendamustine is metabolized and excreted, Dubbelman et al.30 concluded that bendamustine is not expected to accumulate in patients with renal or hepatic impairment given its very short half-life and dosing schedule. Although the study by Dubbelman et al. and others31,32 suggest that the pharmacokinetics of bendamustine are not affected by renal impairment and might therefore be well tolerated in patients with renal insufficiency, the product monograph does not suggest use of the drug in that setting. Consequently, physicians and pharmacists have to assess patients case by case.
6. SUMMARY
Bendamustine is a chemotherapeutic agent with a unique structure and mechanism of action. European experience with bendamustine dating back to the first tests in multiple myeloma in 1969, together with recent work by Rummel et al. and Knauf et al., has provided extensive data on the efficacy and safety of bendamustine in hematologic cancers. The studies hold important implications for the treatment of patients with inhl and cll. The increased efficacy of br over r-chop in inhl and of bendamustine over chlorambucil in cll means that patients have a much lower tumour burden. The typically rapid reduction in the tumour burden is among the distinguishing aspects of bendamustine treatment. The lower toxicity profile includes a reduced frequency of neutropenia and infections (observed with br in patients with inhl). Lower rates of neutropenia and infections lower the risk of serious complications for patients and leads to lower exposure to supportive hematopoietic growth factors and antibiotics. In addition to those efficacy and safety benefits, patients are also likely to experience an improvement in quality of life for the duration of their treatment.
As bendamustine becomes more widely used, information about its safe administration and management of the associated aes becomes crucial to the care and effective treatment of patients with inhl and cll. That information includes standardizing the infusion rate at 60 minutes to reduce the toxicity associated with a high peak plasma concentration, administering pre-medications to control irrs and nausea, hydrating patients to minimize fatigue, and using free-flowing saline at the closest port to avoid phlebitis.
7. ACKNOWLEDGMENTS
Lundbeck Canada supported the development of this paper and the medical writing services provided by Wissam Assaily of New Evidence.
8. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
9. REFERENCES
- 1.Lundbeck Canada . Treanda (bendamustine hydrochloride for injection) Montreal, QC: Lundbeck Canada; 2012. [product monograph] [Google Scholar]
- 2.United States, Department of Health and Human Services, Food and Drug Administration (fda) Bendamustine hydrochloride [Web page] Silver Spring, MD: FDA; 2010. [Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm129215.htm; cited April 1, 2013] [Google Scholar]
- 3.Gandhi V. Metabolism and mechanisms of action of bendamustine: rationales for combination therapies. Semin Oncol. 2002;29:4–11. doi: 10.1053/sonc.2002.34872. [DOI] [PubMed] [Google Scholar]
- 4.Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48(suppl 1):S12–23. doi: 10.1053/j.seminhematol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Rummel M. eCancerTV. Bristol, U.K.: Cancer Intelligence; 2011. The history of bendamustine [Web video] [Available at http://ecancer.org/video/839/the-history-of-bendamustine.php; cited April 5, 2013] [Google Scholar]
- 6.Tageja N. Bendamustine: safety and efficacy in the management of indolent non-Hodgkins lymphoma. Clin Med Insights Oncol. 2011;5:145–56. doi: 10.4137/CMO.S6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rummel MJ, Gregory SA. Bendamustine’s emerging role in the management of lymphoid malignancies. Semin Hematol. 2011;48(suppl 1):S24–36. doi: 10.1053/j.seminhematol.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus chop plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 9.Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab in patients with relapsed follicular, indolent and mantle cell lymphomas—final results of the randomized phase iii study nhl 2-2003 on behalf of the stil (Study Group Indolent Lymphomas, Germany) [abstract 856] Blood. 2010;116 [Google Scholar]
- 10.Pan-Canadian Oncology Drug Review (pcodr) pCODR Expert Review Committee (pERC) Final Recommendation [for bendamustine hydrochloride in indolent non-Hodgkin lymphoma] Toronto, ON: pCODR; 2012. [Available online at http://www.pcodr.ca/idc/groups/pcodr/documents/pcodrdocument/pcodr-treandanhl-fn-rec.pdf; cited April 2, 2013] [Google Scholar]
- 11.Burke JM, Van der Jagt RH, Kahl BS, et al. Differences in quality of life between bendamustine plus rituximab compared with standard first-line treatments in patients with previously untreated advanced indolent non-Hodgkin’s lymphoma or mantle cell lymphoma [abstract 155] Blood. 2012;120 [Google Scholar]
- 12.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase iii randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 13.Knauf WU, Lissitchkov T, Aldaoud A, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase iii trial. Br J Haematol. 2012;159:67–77. doi: 10.1111/bjh.12000. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Wendtner CM, Pieper A, et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk. 2010;10:21–7. doi: 10.3816/CLML.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH, Tremmel L. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:452–7. doi: 10.3816/CLML.2010.n.079. [DOI] [PubMed] [Google Scholar]
- 16.Owen JS, Melhem M, Passarell JA, D’Andrea D, Darwish M, Kahl B. Bendamustine pharmacokinetic profile and exposure–response relationships in patients with indolent non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol. 2010;66:1039–49. doi: 10.1007/s00280-010-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tombleson RL, Ho V, Sokol L, Pinilla J, Wetzstein GA. Optimizing premedications in the prevention of bendamustine infusion-related reactions. Cancer Control. 2012;19:245–7. doi: 10.1177/107327481201900309. [DOI] [PubMed] [Google Scholar]
- 18.Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14:E10–21. doi: 10.1188/10.CJON.E10-E21. [DOI] [PubMed] [Google Scholar]
- 19.Blumel S, Goodrich A, Martin C, Dang NH. Bendamustine: a novel cytotoxic agent for hematologic malignancies. Clin J Oncol Nurs. 2008;12:799–806. doi: 10.1188/08.CJON.799-806. [DOI] [PubMed] [Google Scholar]
- 20.United States, National Institutes of Health, Cancer Therapy Evaluation Program (ctep) Common Toxicity Criteria (CTC). Ver. 2.0. Bethesda, MD: CTEP; 1999. [Available online at: http://www.eortc.be/services/doc/ctc/ctcv20_4-30-992.pdf; cited April 12, 2013] [Google Scholar]
- 21.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue. Fort Washington, PA: NCCN; 2013. Ver. 1.2013. [Available online at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (free registration required); cited April 3, 2013] [Google Scholar]
- 22.Evans M. Cancer Related fatigue [Web video] Toronto, ON: Michael Evans and Mercury Films; 2013. [Available at: http://www.youtube.com/watch?v=YTFPMYGe86s; cited May 1, 2013] [Google Scholar]
- 23.American Society of Health-System Pharmacists . Bendamustine Injection [Web page] Bethesda, MD: MedLine Plus; 2009. [Available at: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a608034.html; cited August 20, 2013] [Google Scholar]
- 24.Drugs.com . Bendamustine [Web page] Auckland, NZ: Drugs.com; 2013. [Available at: http://www.drugs.com/mtm/bendamustine.html; cited August 20, 2013] [Google Scholar]
- 25.The Scott Hamilton Cares Initiative . Bendamustine [Web page] Cleveland, OH: The Scott Hamilton Cares Initiative; n.d. [Available at: http://chemocare.com/chemotherapy/drug-info/bendamustine.aspx; cited August 20, 2013] [Google Scholar]
- 26.Multinational Association of Supportive Care in Cancer (mascc) MASCC/ESMO Antiemetic Guideline 2013. Hillerød, Denmark: MASCC; 2013. [Available online at: http://www.mascc.org/assets/documents/mascc_guidelines_english_2013.pdf; cited April 8, 2013] [Google Scholar]
- 27.NursingTimes.net . Phlebitis: treatment, care and prevention [Web article] London, U.K.: EMAP Publishing; 2011. [Available at http://www.nursingtimes.net/nursing-practice/clinical-zones/infection-control/phlebitis-treatment-care-and-prevention/5034782.article; cited April 12, 2013] [Google Scholar]
- 28.Registered Nurses’ Association of Ontario (rnao) Care and Maintenance to Reduce Vascular Access Complications. Toronto, ON: RNAO; 2005. [Available online at: http://rnao.ca/sites/rnao-ca/files/Care_and_Maintenance_to_Reduce_Vascular_Access_Complications.pdf; cited March 28, 2013] [Google Scholar]
- 29.Darwish M, Megason GC, Bond M, et al. Pharmacokinetics (pk) of bendamustine in pediatric patients with relapsed/refractory acute leukemia [abstract 9547] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/97053-114; cited December 31, 2013] [Google Scholar]
- 30.Dubbelman AC, Rosing H, Darwish M, et al. Pharmacokinetics and excretion of 14C-bendamustine in patients with relapsed or refractory malignancy. Drugs R D. 2013;13:17–28. doi: 10.1007/s40268-012-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordstrom BL, Knopf KB, Teltsch D, Engle R, Beygi H, Sterchele JA. Retrospective safety assessment of bendamustine in patients with renal impairment [abstract e13081] J Clin Oncol. 2012;30 doi: 10.3109/10428194.2013.836600. [Available online at: http://meetinglibrary.asco.org/content/98848-114; cited December 31, 2013] [DOI] [PubMed] [Google Scholar]
- 32.Ponisch W, Andrea M, Wagner I, et al. Successful treatment of patients with newly diagnosed/untreated multiple myeloma and advanced renal failure using bortezomib in combination with bendamustine and prednisone. J Cancer Res Clin Oncol. 2012;138:1405–12. doi: 10.1007/s00432-012-1212-4. [DOI] [PubMed] [Google Scholar]