TABLE I.
Summary of key input parameters
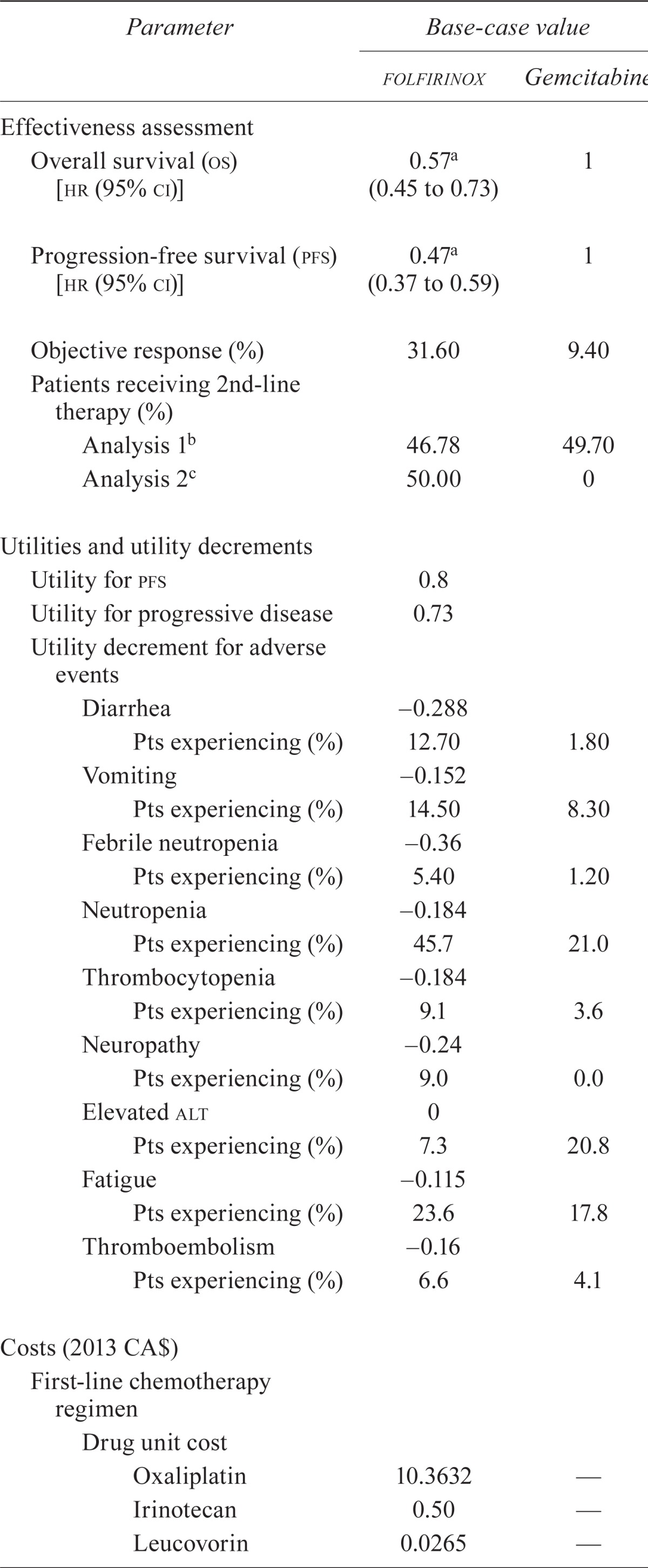
| Parameter |
Base-case value
|
|
|---|---|---|
| folfirinox | Gemcitabine | |
| Effectiveness assessment | ||
| Overall survival (os) [hr (95% ci)] | 0.57a (0.45 to 0.73) | 1 |
| Progression-free survival (pfs) [hr (95% ci)] | 0.47a (0.37 to 0.59) | 1 |
| Objective response (%) | 31.60 | 9.40 |
| Patients receiving 2nd-line therapy (%) | ||
| Analysis 1b | 46.78 | 49.70 |
| Analysis 2c | 50.00 | 0 |
| Utilities and utility decrements | ||
| Utility for pfs | 0.8 | |
| Utility for progressive disease | 0.73 | |
| Utility decrement for adverse events | ||
| Diarrhea | −0.288 | |
| Pts experiencing (%) | 12.70 | 1.80 |
| Vomiting | −0.152 | |
| Pts experiencing (%) | 14.50 | 8.30 |
| Febrile neutropenia | 0.36 | |
| Pts experiencing (%) | 5.40 | 1.20 |
| Neutropenia | −0.184 | |
| Pts experiencing (%) | 45.7 | 21.0 |
| Thrombocytopenia | −0.184 | |
| Pts experiencing (%) | 9.1 | 3.6 |
| Neuropathy | −0.24 | |
| Pts experiencing (%) | 9.0 | 0.0 |
| Elevated alt | 0 | |
| Pts experiencing (%) | 7.3 | 20.8 |
| Fatigue | −0.115 | |
| Pts experiencing (%) | 23.6 | 17.8 |
| Thromboembolism | 0.16 | |
| Pts experiencing (%) | 6.6 | 4.1 |
| Costs (2013 CA$) | ||
| First-line chemotherapy regimen | ||
| Drug unit cost | ||
| Oxaliplatin | 10.3632 | — |
| Irinotecan | 0.50 | — |
| Leucovorin | 0.0265 | — |
| Fluorouracil | 0.004 | — |
| Gemcitabine | — | 0.07 |
| Total drug costs per cycle | 1,737.18 | 122.50 |
| Relative dose index (rdi) | 0.8 | 1.0 |
| rdi adjusted drug cost | 1,389.74 | 122.50 |
| Co-medication drug costs | 52.92 | 10.90 |
| Monitoring and administration cost | 190.55 | 67.56 |
| Total cost per cycle | 1,633.21 | 200.96 |
| Second-line platinum-based chemotherapy regimen (per cycle) | ||
| folfox | 1,574.08 | |
| gemox | 1,936.06 | |
| 5-Fluorouracil/cisplatin | 92.31 | |
| folfirinox | 1,737.18 | |
| Weighted averaged | 1,378.03 | |
| Costs to treat adverse events (grade 3 or 4)e | ||
| Hospitalization for diarrheaf | 7,867 | |
| Hospitalization for vomitingg | 3,467 | |
| Hospitalization for thromboembolismh | 8,451 | |
| Hospitalization for febrile neutropeniai | 6,324 | |
| Cost of g-csf [2013 CA$ (% pts treated)] | ||
| Analysis 1 | 4,411j (42.5) | 4,411j (5.3) |
| Analysis 2 | — (0) | — (0) |
p < 0.001.
Based on accord intention-to-treat patient populations (n=171).
Based on current Ontario treatment patters and the hypothetical model cohort (n=1000).
Based on 56.0%, 20.0%, 18.7%, and 5.3% for folfox, gemox, 5-fluorouracil/cisplatin, and folfirinox respectively.
Percentage assumed based on clinical expert opinion.
Cost based on the assumption that 50% of patients with diarrhea are hospitalized12.
Cost based on the assumption that 10% of patients with vomiting are hospitalized12.
Cost based on the assumption that 25% of patients with thromboembolism are hospitalized12.
Cost based on the assumption that 100% of patients with febrile neutropenia are hospitalized13.
Represents the cost of granulocyte colony–stimulating factor for a mean of 21.44 days at a dose of 0.300 mg daily14.
folfirinox = 5-flourouracil, leucovorin, oxaliplatin, irinotecan; hr = hazard ratio; ci = confidence interval; pts = patients; alt = alanine aminotransferase; folfox = 5-flourouracil, leucovorin, oxaliplatin; gemox = gemcitabine, oxaliplatin; g-csf = granulocyte colony–stimulating factor.
