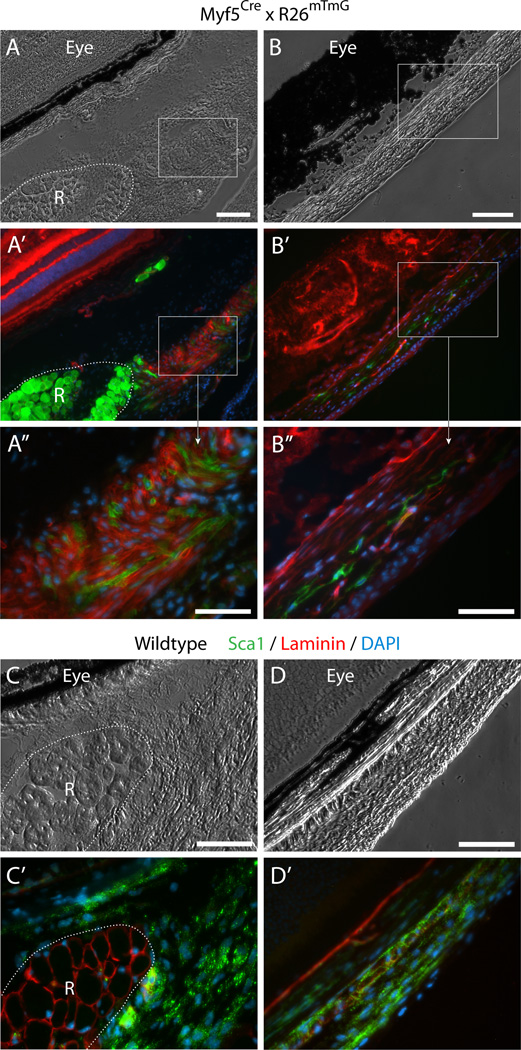
Fig. 4. Histological detection of Myf5Cre-driven GFP+ cells in connective tissues of periocular preparations.
(A–B”) Cross sections from Myf5Cre × R26mTmG mice analyzed for GFP and Tomato fluorescence. (C–D’) Cross sections from wildtype mice labeled by double-immunofluorescence with anti-Sca1 and anti-lamininSca1 marked cells within the periocular connective tissues and laminin identified the basal lamina of individual myofibers, delineating the rectus muscle. Unlike the straightforward Sca1 immunostaining of wildtype tissue, Sca1 immunolabeling of tissue sections from the Myf5Cre × R26mTmG reporter line required using the far-red or the blue channels, but these did not provide effective detection. First, as the red fluorescence bleeds into the far-red channel, and as both the Tomato and Sca1 signals are localized to the cytoplasmic membrane, determination of Sca1 staining in far-red is unreliable. Second the use of a number of secondary antibodies conjugated with different types of blue fluorophores yielded only extremely faint label with a low signal-to-noise ratio. Images shown in the figure are from cross sections at the level of the eyeball, either (A-A”) and (C-C’) posterior, or (B-B”) and (D-D’) anterior to the attachment of the EOM to the eye. R, rectus muscle. In all images DAPI staining is shown in blue. Scale bars in (A) and (B), 100µm, and in (A”), (B”), (C) and (D), 50µm.