Abstract
Ischemia reperfusion injury (IRI) contributes to partial flap and solid organ transplant failure. Heme-oxygenase 1 (HO-1) is an inducible, cytoprotective enzyme which protects against IRI in solid organ transplant models. Heme arginate (HA), a HO-1 inducer, is a promising, translatable, preconditioning agent. This study investigated the effects of preconditioning with HA on the clinical outcome of a myocutaneous IRI model. Forty male Lewis rats were randomized to intravenously receive 1) Control-NaCl, 2) HA, 3) HA and tin mesoporphyrin (SnMP), a HO-1 inhibitor; and 4) SnMP alone. Twenty-four hours later, an in situ transverse rectus abdominis myocutaneous flap was performed under isoflurane anesthesia. Viability of flaps was measured clinically and by laser-Doppler perfusion scanning. In vitro work on human epidermal keratinocytes (HEKa) assessed the effects of HA, SnMP, and the iron chelator desferrioxamine on 1) cytotoxicity, 2) intracellular reactive oxygen species (ROS) concentration, and 3) ROS-mediated DNA damage. In contrast to our hypothesis, HA preconditioning produced over 30% more flap necrosis at 48 h compared with controls (P = 0.02). HA-containing treatments produced significantly worse flap perfusion at all postoperative time points. In vitro work showed that HA is cytotoxic to keratinocytes. This cytotoxicity was independent of HO-1 and was mediated by the generation of ROS by free heme. In contrast to solid organ data, pharmacological preconditioning with HA significantly worsened clinical outcome, thus indicating that this is not a viable approach in free flap research.
Keywords: free tissue transfer, heme arginate, heme-oxygenase-1, ischemia reperfusion injury, myocutaneous flap
free tissue transfer is the gold standard of management in reconstructive surgery when local options are unavailable or unsuitable. This type of surgery is widely and routinely performed following oncological resection and major trauma. Ischemia-reperfusion injury (IRI) is unavoidable in this surgery, has no treatment, and is accepted as a major cause of partial flap failure (36, 87, 100, 107, 108). Preconditioning strategies train tissue to withstand better a known, future insult. The elective nature of free tissue transfer provides a suitable window of opportunity for a pharmacological intervention strategy to precondition tissue.
The flap is rendered ischemic following transection of the vascular pedicle until blood flow is reestablished by microanastomosis. ATP stores are depleted impairing homeostatic pumps leading to a rise in intracellular sodium and calcium concentrations (24). If ischemia is prolonged; the deranged electrochemical gradients and low pH will overwhelm cellular homeostatic mechanisms and lead to cell necrosis (12, 90). Reperfusion is the only means to halt this inevitable progression. However, reperfusion also permits the influx of inflammatory cells and mediators, which initially worsen the injury (13). This damage is primarily mediated by reactive oxygen species (ROS) produced from endothelial cells (71) and neutrophils (56). ROS cause opening of the mitochondrial permeability transition pore on the inner mitochondrial membrane and result in induction of proapoptotic pathways (109). Intravital microscopy has shown that the net effect of these changes is altered hemodynamics within the microcirculation (79). If severe, this can lead to inadequate perfusion and subsequent necrosis.
Heme-oxygenase 1 (HO-1) is a key homeostatic inducible enzyme that has an important role in IRI. HO-1 breaks down heme to carbon monoxide, free iron, and biliverdin. Biliverdin is rapidly reduced by cytosolic biliverdin reductase to bilirubin (10). In both humans and rodents, HO exists in two main isoforms HO-1 the inducible (32-kDa protein) and HO-2 the constitutively expressed, noninducible (36-kDa protein) encoded by HMOX-1 and HMOX-2, respectively (32). The HO-1 enzymatic pathway is highly conserved among mammals and is induced in response to disparate cell stressors (32). HO-1 induction reduces apoptosis (35), provides defense against oxidative stress (72), suppresses thrombus formation (60, 97), and decreases vasoconstriction (21). CO alone has been shown to replicate these effects on apoptosis (8) and inflammation (69). Bilirubin is a powerful antioxidant and may be the means by which HO-1 induction protects against oxidative stress (7, 9, 89). In addition, HO-1 protects endothelial cells against complement-mediated injury through induction of decay-accelerating factor (49).
Inherited deletion of HO-1 in humans is often embryonically lethal and in those few that do survive there is evidence of cardiovascular disease, inflammation, and markedly reduced life span (46, 50, 74, 88). Furthermore, the products of the HO-1 pathway confer cytoprotection in cardiac (11, 113), renal (27, 96), liver (19, 83), lung (106, 117), and intestinal (3) models of IRI. Polymorphisms within the HO-1 promoter (leading to variable HO-1 expression) can affect susceptibility and severity of a number of diseases, including IRI (15, 61, 112). In addition, other cytoprotective pathways, such as interleukin-10 (14, 59), mediate their effects through HO-1 induction—a process coined the HO-1 therapeutic amplification funnel (4). As all three products are biologically active, are produced simultaneously, and may have complex feedback effects with each other and on HO-1 expression, it is difficult to establish the precise role of each component in isolation.
Heme arginate (HA) is a drug containing human hemin stabilized in arginine. It is licensed in Europe for the treatment of acute porphyria. A human trial of the effects of HA on healthy volunteers reported a 75-fold increase in plasma heme concentration, compared with controls (20). This remained elevated for 48 h and was still over 25 times greater than the baseline value at 24 h. It was associated with strong up-regulation of HMOX-1 expression; increased HO-1 protein level, and HO-1 activity in peripheral blood monocytes (20). HA up-regulates HO-1 through heme by modulating HMOX-1 transcription. Bach-1 is a transcription repressor and functions as the sensor and effector of heme and represses HMOX-1 transcription (39). Heme binds the Bach-1 repressor through multiple heme regulatory motifs causing conformational changes that prevent its repressor actions (38, 66). Heme binding to Bach-1 leads to its exportation from the nucleus into the cytosol (93). Heme then regulates the polyubiquination and degradation of Bach-1 (115). The overall effect of heme's action on Bach-1 is derepression of Bach-1 target genes, including HMOX-1 (91). HA was selected as it is a potent inducer of HO-1 and has successfully been used to ameliorate the following IRI models: intestine (3), lung (106), liver (47, 53), and kidney (28). HA also has a well-established safety profile in humans (1, 20), permitting its translation into clinical trials.
MATERIALS AND METHODS
Reagents
HA was obtained from Orphan Europe (Oxford, UK). Tin mesoporphyrin (SnMP) was obtained from Frontier Scientific (via Inochem, Carnforth, Lancashire, UK) and dissolved in 0.1 M NaCl, at pH 7.4. Hydrogen peroxide (H2O2) and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetylester-5-(and-6)-(CM-H2DCFDA) were purchased from Invitrogen (Carlsbad, CA). Methylthiazolyldiphenyl-tetrazolium bromide (MTT), desferrioxamine (DF), SDS, citric acid, acetone, and radioimmunoprecipitation assay (RIPA) were obtained from Sigma-Aldrich (Gillingsworth, UK). ViaLight plus kit was obtained from Lonza (Nottingham, UK). 8-Hydroxy-2′-deoxguanosine (8-OHdg) ELISA was obtained from Trevigen (Gaithersburg, MD).
All cells and cell culture materials were purchased from Invitrogen unless otherwise stated. Adult human epidermal keratinocytes (HEKa; C-005–5C) were maintained in serum-free, Epilife medium (M-EPI-500-CA) human keratinocyte growth supplement kit (S-001–5) and penicillin (100 U/ml; PAA Laboratories, Somerville, UK) streptomycin (100 μg/ml PAA) at 37°C, 5% CO2. Tissue culture plastics were obtained from Costar (Loughborough, UK). Dulbecco's phosphate buffered saline (DPBS) without calcium and magnesium and trypsin-EDTA were purchased from PAA.
Animals
Lewis rats aged 6–8 wk weighing 250–350 g were purchased from Harlan and housed in the animal husbandry unit at the University of Edinburgh in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The procedures were performed under UK Home Office licenses PPL 60/4045 and PIL 60/12484. Animals were randomly assigned to one of four preconditioning groups using the random sequence generator (http://www.random.org) to receive a 1 ml, intravenous injection of 1) Control = NaCl, 2) HO-1 inducer = HA (30 mg/kg), 3) HO-1 inducer + inhibitor HA (30 mg/kg) + SnMP (40 μmol/kg); and 4) HO-1 inhibitor alone-SnMP (40 μmol/kg). A preliminary cohort of sixteen rats was used to establish efficacy of this dose of HA in up-regulating HO-1, but these rats did not undergo surgery. These animals were culled and tissue harvested 24 h after administration of the preconditioning agents. Once this was established, a larger cohort of 40 rats were then randomly assigned an order to undergo the surgical procedure 24 h after the pharmacological preconditioning treatments were administered.
Operative Procedure and Perfusion Scanning
The animals were anesthetized using inhalational isoflurane, the anterior abdominal wall was depilated, and chlorhexidine (Hibiscrub, Miller Medical, Newport, UK) was applied. All surgical procedures were performed under aseptic techniques. A 4 × 4 cm, centrally placed flap with the upper margin 0.8 cm below the xiphisternum was designed on the anterior abdominal wall. A laser-Doppler imaging (LDI) scanner (Moor LD12, Moor Instruments, Essex, UK) was employed to assess preoperative perfusion (94, 95). A transverse rectus abdominis myocutaneous flap was raised based on the left superior, deep, epigastric vascular pedicle (23). The left rectus abdominis muscle was tied off caudally, and the myocutaneous perforators coming through the right rectus abdominis muscle were cauterized. Ellipses of 0.5-mm-thick, flexible silicone sheeting (J-flex, London, UK) were placed and secured with 6–0 vicryl rapide (Miller Medical) under the fasciocutaneous portions of the flap to prevent them taking as a full thickness graft (29). The left, deep, superior epigastric vascular pedicle was then exposed. Single, Acland, atraumatic clamps (Mercian Surgical, Bromsgrove, UK) were applied to both the artery and vein for 30 min to reproduce the ischemic injury associated with free flap surgery. This ischemic time point was established in preliminary work to produce a reliable rate of 30% necrosis at 48 h in control animals (data not shown). The clamps were removed and return of flow was confirmed. The cut ends of the rectus muscle were then approximated with 6–0 vicryl, and the flap was sutured in place using subcuticular, 4–0 monocryl. A postoperative LDI scan was performed, and the rat returned to an incubator at 37°C for 1 h. The LDI scans were repeated at 24 and 48 h postoperatively. Buprenorphine (0.04 mg/kg sc) was administered when clamps were applied for postoperative analgesia. Warmed, sterile saline (1 ml·kg−1·h−1 sc) was administered hourly to maintain intravascular fluid volume.
At the time of each LDI scan, a high-resolution image was obtained. These images were used to calculate percentage area skin survival at 48 h using Image J (84). The Moor Instruments Software supplied with the LDI scanner was used to calculate the average perfusion of the flap (95).
The animals were culled after the 48 h LDI scan, and the tissue was collected for protein analysis; histological analysis (fixed in Methyl Cornoy's solution) and embedded in Tissue-Tek OCT (Sakura, Alphen aan den Rijn, The Netherlands) and frozen for immunofluorescent staining.
Blood Pressure Assessment
Six, 6–12-wk-old, male, Lewis rats were housed in groups of three and habituated over the course of 10 days to have their systolic blood pressure measured by the tail-cuff method using a computer-assisted oscillatory detection device (TSE Systems, Bad Homburg, Germany), as previously described (51). One group received 1 ml intravenous sterile 0.9% NaCl and the other 30 mg/kg HA (1 ml) After 24 h, each rat underwent 8 independent blood pressure recordings, and the average systolic blood pressure was calculated. Seventy-two hours after the original set of injections, the rats were crossed-over into the other treatment arm of the study, and the procedure was repeated. The average blood pressure of six rats for each treatment was then calculated, and their means were compared using the unpaired t-test.
Cells and Cell Culture
HEKa cells were seeded in 96-well plates for cytotoxicity assays and 25 cm2 flask for the CM-H2CFDA assay and cultured to confluency. The medium was then replaced with the seven preconditioning media (10 wells/condition per n) for a further 24 h at 37°C, 5% CO2. The conditions were 1) control medium, 2) 10 μmol HA, 3) 5 μmol SnMP + 10 μmol HA; 4) 10 μmol HA + 500 μmol DF; 5) 10 μmol HA + 5 μmol SnMP + 500 μmol DF; 6) 5 μmol SnMP; and 7) 500 μmol DF.
Cytotoxicity
MTT assay.
The preconditioning media were removed and replaced with fresh media. Then 10% (vol/vol) of 5 mg/ml MTT was added to each well and incubated for 4 h at 37°C. The MTT-containing media were then removed and 10% SDS (pH 3) added to each well and incubated overnight at 37°C on an agitating platform. The optical density (OD) was then read at 570 nm on a Biotek Synergy HF plate reader and using Gen 5 microplate reader software. Percentage cell viability was calculated as follows: (OD treated cells/OD control cells) × 100.
Vialight plus ATP assay.
This is a bioluminescent measurement of ATP production from living cells based on the conversion of luciferin to oxyluciferin and light, in the presence of luciferase and ATP. A rapid decrease in cytoplasmic ATP is seen following cell injury (18). Change in ATP level can, therefore, be used as a marker of cytotoxicity. The kit was used as per manufacturer's guidelines.
Measurement of Intracellular ROS Accumulation—CM-H2DCFDA Assay
The redox state of the cells was assessed by measuring the increase in fluorescence following treatment with CM-H2DCFDA, as previously described (25, 57, 65, 67). In brief, cells were incubated in DPBS containing 10 μM CM-H2DCFDA for 30 min at 37°C. Cells were then collected by trypsinization, centrifuged, and resuspended in DPBS. A total of 5,000 events per condition were assessed by flow cytometry on a LSR Fortessa scanner (BD Biosciences, Oxford, UK) and analyzed using FlowJo Version X software (www.FlowJo.com). Accumulation of intracellular 2′7′-dichlorodihydrofluorescein (DCF) was detected as an increase in fluorescence at 530 nm following excitation at 485 nm.
Measurement of DNA Damage Mediated by Oxidative Stress: 8-OHdg ELISA
Oxidative stress refers to a physiological state in which the intrinsic antioxidant defenses of the cell are overwhelmed by local generation of ROS. DNA is a common target of these ROS resulting in genome instability. The production of 8-OHdg is a highly specific marker of oxidative stress (58). This ELISA was performed as per manufacturer's guidelines to quantitatively assess the amount of 8-OHdg in the cell samples.
Immunohistochemistry
Antibodies.
Rabbit anti-HO-1 (1:200) was from Enzo Life Sciences (Exeter, UK). Mouse anti-CD68 (pan-macrophage marker; 1:250) was from AbD Serotec (Kidlington, UK). Goat anti-CD206 (type 2 macrophage marker; 1:200) was from Santa Cruz Biotechnology (Heidelberg, Germany). Rabbit anti-myeloperoxidase (MPO; neutrophil marker; 1:200) was from DAKO (Ely, UK). Biotinylated secondary antibodies were purchased from Vector Laboratories (Peterborough, UK).
Immunohistochemical analysis was performed on 5-μm sections prepared from Methyl Cornoy's fixed, paraffin-embedded tissue. Slides were incubated in 2% H2O2 for 15 min at room temperature (RT) to inhibit endogenous peroxidase activity. Incubated in serum-free protein block for 10 min at RT (X0909: DAKO, Ely, UK) followed by incubation with the primary antibody overnight at 4°C. Following PBS washes, the slides were incubated with a biotinylated secondary antibody for 30 min at RT. Following further PBS washes; 150 μl of Vector Laboratories R.T.U Elite ABC reagent (Peterborough, UK) was added to each slide for 30 min at RT. Following PBS washes; diaminobenzidine (DAB; DAKO, Ely, UK) was used to visualize staining and hematoxylin employed as a counterstain. Ten high-powered field images were taken of the epidermis/dermis, subcutis, and muscle of these sections, and the average number of positive cells was calculated. This was repeated for four subjects per preconditioning group. Images were captured using an Olympus Provis AX70 microscope with an Axio Cam HRC Zeiss Camera and Axiovision Release 4.8.2 software.
Immunofluorescence
Antibodies.
Rabbit anti-HO-1 (1:50) was obtained from Enzo Life Sciences (Exeter, UK). Mouse anti-CD31 (1:50) was obtained from BD Biosciences (Oxford, UK). Mouse anti-CD68 (1:100) was obtained from AbD Serotec (Kidlington, UK). Goat anti-CD206 (1:100) was obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Mouse anti-tryptase (1:100) was obtained from Abcam (Cambridge, UK). Mouse anti-α smooth muscle actin (α-SMA; 1:200) was obtained from Invitrogen (Paisley, UK). Goat anti-mouse Alexa Fluor-488; chicken anti-goat Alexa Fluor-488 and donkey anti-rabbit Alexa Fluor-555 were obtained from Invitrogen (Paisley, UK).
Fresh frozen sections were stained for HO-1 and primary antibodies to CD31 an endothelial cell marker; CD68, a pan-macrophage marker; CD206, for type 2 macrophages; tryptase for mast cells; and α-smooth muscle actin (α-SMA) for pericytes (17, 43, 86). In brief, slides were air-dried for 30 min at room temperature; permeabilized for 5 min in glacial acetone (−20°C); washed in PBS and serum-free protein blocked (X0909: DAKO, Ely, UK) for 1 h at RT. Slides were then incubated in the primary antibodies overnight at 4°C, washed in PBS, and incubated in the fluorochrome-conjugated secondary antibodies for 1 h at RT at 1:200. The slides were washed in PBS and mounted in ProLong Gold mounting media with 4′,6-diamidino-2-phenylindole (DAPI) as a nuclear counter stain (Invitrogen; Paisley, UK).
In addition, mast cells were stained using avidin conjugated to 1:250 Alexa Fluor-488 (Invitrogen A21370), as described by Veerappan et al. (101).
Images were captured using a confocal Leica TCS SP5 system and Leica application suite software (Leica, Milton Keynes, UK).
Histological Classification of Injury
There is no accepted histological classification of injury seen following IRI in myocutaneous flaps. Hematoxylin and eosin (H&E)-stained slides were examined by the author and resident veterinary pathologist (Dr. J Baily). The characteristics of severe injury are summarized in Table 1. Tiled images of the sections were taken using a Nikon Eclipse E800 microscope, QICAM Q Imaging Fast 1394 camera and Image Pro Plus Media cybernetics (version 7.0) software. The author was blinded to the treatment group (control, HA, HA + SnMP, and SnMP) and type of surgical procedure/section (normal, sham, Zone IV-IRI, Zone I-IRI). Each section was assessed, and the percentage total area/total number of structures were assessed and scored 0–4 on each of the criteria shown in Table 1, and a composite injury score was obtained (0–16). Sections were excluded if they failed to demonstrate the three sections of the skin: epidermis; dermis, and subcutis. A score of 0 meant that these features were entirely absent. If present in <25% of the area/total number of those structures in section, it would score 1. If observed in 25 to <50%, it would score 2. If observed in 50 to <75%, it would score 3, and if observed in >75% of the section, it would score 4. Therefore, the maximum injury score for a section is 16 and the minimum injury score is 0.
Table 1.
Histological classification of injury
| Epidermis | Dermis/Subcutis | ||
|---|---|---|---|
| Nuclear change (pyknosis, karyohexis, and karyolysis) or denuding of the epithelium | Adnexal necrosis: follicular epithelial cells and sebocytes appear shrunken with hyperacidophilic, hyperchromatic nuclei. Vacuolated appearance of sebocytes. | Collagen necrosis: hyperacidophilia and loss of fibrilliary detail resulting in the formation of a deep pink amorphous layer. Associated nuclei frequently pyknotic. | Edema/hemorrhage: Hemorrhage is identified as extravasated erythrocytes. Interstitial edema is seen as proteinaceous fluid that is pale pink, homogenous and expands extracellular spaces. |
Hematoxylin -and -eosin-stained slides were assessed from: normal skin; skin following sham surgery; skin from Zone IV; and skin from Zone I post-IRI. The following characteristics were found in severely injured tissues. This formed the basis of a composite scoring system for the degree of histological injury.
HO-1 Activity Assay
The method used for the determination of HO-1 activity via bilirubin formation follows the protocol published by McNally et al. (63). HO-1 activity was measured as picomoles of bilirubin formed per milligram of liver protein lysate per hour.
Protein Lysates—Solid Organs
Frozen tissue was suspended in 500 μl of RIPA buffer with protease inhibitor (Mini Complete Roche, Welwyn Garden City, UK). The samples were homogenized using the Qiagen Tissue Lyser at 25 Hz for 4 min. Samples were centrifuged at 10,000 g for 5 min at 4°C. A Bio-Rad (Bio-Rad, Hertfordshire, UK) protein assay was performed on the supernatant to establish protein concentration.
Western Blot Analysis
Proteins were separated by SDS-PAGE (10% Tris·HCl), transferred to a polyvinylidene fluoride membrane from Merck Millipore (Watford, UK), and exposed to blocking buffer followed by incubation in the primary antibodies: rabbit anti-HO-1 (1:2,000) from Enzo Life Sciences (Exeter, UK), and rabbit anti-β actin (1:5,000) from Abcam (Cambridge, UK). A horseradish peroxidase-conjugated secondary was then used: goat anti-rabbit (1:2,000 from DAKO, Ely UK). Protein bands were visualized using SuperSignal West Pico chemiluminescent substrate, an enhanced chemiluminescent (ECL) system from Thermo Fisher Scientific (Northumberland, UK) in conjunction with the VersaDoc imaging system and Quantity One image capture software, both from Bio-Rad (Hemel Hempstead, UK).
Statistical Analysis
Data are given as means ± SE unless otherwise indicated. The effects of the preconditioning treatments was assessed by one-way ANOVA, normality confirmed by Brown-Forsythe test, and completed using Tukey's post hoc test to compensate for multiple comparisons. All statistical calculations were computed using GraphPad PRISM software version 6 (GraphPad Software, San Diego, CA), and significance was determined at P < 0.05.
RESULTS
Effectiveness of HA on HO-1 Induction and Bioactivity
Western blot confirmed that parenteral administration of HA resulted in systemic upregulation of HO-1 in all tissues assessed (Fig. 1). HA treatment also increased HO-1 bioactivity (Fig. 1) in homogenized liver samples. One-way ANOVA comparing the effects of the preconditioning treatments on HO-1 bioactivity was significant, P < 0.0001. HA preconditioning increased HO-1 bioactivity ∼20-fold (2,913 ± 174.7) compared with controls (159 ± 1.00). This increase in HO-1 bioactivity was abrogated by the coadministration of SnMP (208 ± 52.05). Furthermore, administration of SnMP (91.1 ± 14.58) reduced bioactivity by 57% compared with controls.
Fig. 1.

Western blots from skin (A), muscle (B), fat (C), spleen (D), kidney (E), and liver (F). Protein lysates were obtained from tissues harvested 24 h after administration of 0, 5, 15, and 30 mg/kg of HA via intravenous injection. The upper bands are β-actin (42 kDa), a loading control, and the lower bands are HO-1 (32 kDa). Of all the tissues assessed, only the skin and spleen show baseline HO-1 activity. A dose-related increase in HO-1 was found in all the tissues assessed. G: HO-1 activity assay results (n = 3 per group). Animals received intravenous preconditioning with control = NaCl; HA (30 mg/kg); heme arginate (HA) + tin mesoporphyrin (SnMP) (30 mg/kg HA and 40 μmol/kg SnMP); and SnMP (40 μmol/kg) alone. After 24 h, the animals were culled, tissue was harvested, and samples were prepared from homogenized livers. HA administration resulted in a significant increase of HO-1 [F(3, 8) = 228, P < 0.0001; r2 = 0.99]. This activity was abrogated by coadministration of SnMP. Statistically significant differences between preconditioning groups are indicated as follows: ***P < 0.001, as established be one-way ANOVA and Tukey's post hoc test.
Localization of HO-1 in the Skin
Immunohistochemistry was undertaken to establish in which cells HA induced HO-1 expression in the skin (Fig. 2). HO-1-positive cells (Fig. 2, A–D) were seen throughout the epidermis/dermis, muscle, and subcutis in all the preconditioning treatments. The preconditioning treatments did not affect the average number of HO-1-positive cells in the epidermis/dermis or muscle. However, in the subcutis, preconditioning significantly affected the number of HO-1-positive cells compared with controls, P < 0.0001 (see Table 2). In the subcutis, there were significantly more HO-1-positive cells following HA preconditioning: control (9.18 ± 2.69) vs. HA (23.98 ± 2.32), P = 0.0008; HA vs. HA + SnMP (5.06 ± 1.35), P < 0.0001; and HA vs. SnMP (7.83 ± 0.76). These findings correlate with the Western blot data that 1) there is baseline expression in the skin and 2) this can be significantly increased by parenteral administration of HA.
Fig. 2.
A–D: HO-1-positive staining within the skin with ×100 magnification (×400, insets) for control = NaCl (A), HA (30 mg/kg) (B), HA + SnMP (30 mg/kg HA and 40 μmol/kg SnMP) (C), and SnMP (40 μmol/kg) alone (D). E: colocalization of HO-1 (red) and pan-macrophage marker CD68 (green) at ×400 confocal magnification. White arrows indicate cells in which CD68 and HO-1 were colocalized. Blue arrow indicates HO-1-positive cells with no concurrent CD68 staining. F–H: black and white images of the three separate channels, which make up composite image (E): DAPI (F), CD68 (G), and HO-1 (H).
Table 2.
In a preliminary experiment, 16 animals were divided into 4 groups to receive NaCl, HA, HA + SnMP, or SnMP alone
| Preconditioning Treatment | Average Number of Cells per Hpf ± SE |
|---|---|
| Control | 9.18 ± 2.69 |
| HA | 23.98 ± 2.35 |
| HA + SnMP | 5.06 ± 1.35 |
| SnMP | 7.825 ± 0.76 |
Values are expressed as means ± SE of average number of HO-1-positive cells counted per high-powered field (hpf; ×400) in the subcutis. Twenty-four hours later, they were culled, and tissue was harvested for immunohistochemincal analysis. These animals did not undergo transverse rectus abdominis myocutaneous (TRAM) flap surgery. Average of 10 high-powered fields/subject and four subjects per group are shown. One-way ANOVA demonstrated a significant difference in cell count between the preconditioning groups [F (3,12) =19.05, P < 0.0001, r2 = 0.83].
HA, heme arginate; SnMP, tin mesoporphyrin.
The HO-1-positive cells were observed in two main locations in the skin: 1) in close proximity with blood vessels within the subcutis and 2) interspersed between collagen fibrils of the dermis. Colocalization of HO-1 with macrophages (CD68), mast cells (tryptase and avidin), neutrophils (MPO), endothelial cells (CD31), or pericytes (α-SMA) was assessed. HO-1 was not found to colocalize with any of the cell types interrogated except for the pan macrophage marker CD68 (Fig. 2, E–H). Thus, parenteral administration of HA in the rat systemically upregulated biologically active HO-1 and increased HO-1 expression in the skin, particularly, in macrophages. It should be noted that not all HO-1-positive cells (blue arrow in Fig. 2) costained for the pan macrophages marker. HO-1 is, therefore, present in at least one other cell type. These cells may be pericytes, which can be difficult to stain. α-SMA is a known pericyte marker (17, 43, 86). However, not all pericytes are α-SMA-positive. Attempts were made to use different pericyte markers, proteoglycan and platelet-derived growth factor receptor β (NG2 and PDGFRβ, respectively), but this staining was unsuccessful (data not shown).
HA Preconditioning Exacerbates Skin Necrosis and Histological Injury
The model resulted in significant skin necrosis 48 h following reperfusion (Fig. 3). Contrary to our hypothesis, HA pretreatment exacerbated skin necrosis [Control (37.86 ± 5.43) vs. HA (69.76 ± 8.80)], and this effect was not reversed by HO-1 inhibition with SnMP [Control vs. HA + SnMP (85.00 ± 3.61)] (Figs. 3 and 4A). SnMP alone had no effect on the severity of injury. Furthermore, the combination of IRI and preconditioning with HA also led to worse composite histological injury scores in both Zone I and Zone IV (Fig. 4 B–D). HA and SnMP preconditioning led to the most severe injury scores. Interestingly, the mean composite injury values for Zone IV (Fig. 4C) and Zone I (Fig. 4D) are similar, despite there being more obvious clinical injury in Zone IV than Zone I (Fig. 3). Thus, HA treatment led to worse clinical and histological outcome following skin IRI (Fig. 4, B–D).
Fig. 3.
Representative results of necrosis and perfusion 48 h postoperatively. Animals were randomized to receive either Control-NaCl, HA (30 mg/kg), HA + SnMP (30 mg/kg HA + 40 μmol/kg SnMP), and SnMP (40 μmol/kg) alone by intravenous injection. After 24 h, these animals underwent the transverse rectus abdominis myocutaneous (TRAM) procedure. The preconditioning groups were control, HA, HA + SnMP, and SnMP. The upper row are high-resolution images from which percentage area necrosis was calculated. The lower row shows the corresponding laser-Doppler imaging scans. Perfusion is given in perfusion units, and each unit is assigned a color. The scale is shown below the images.
Fig. 4.
A: mean percentage area flap necrosis at 48 h following TRAM flap procedure. A one-way ANOVA between the pretreatment groups on this outcome measure was significant, P < 0.0001. Tukey's post hoc comparison of the mean values of these groups showed a significant increase in flap necrosis in both the HA and HA + SnMP-pretreated groups compared with control and SnMP-pretreated animals as denoted by *P < 0.05, **P < 0.01, and ***P < 0.001; n = 10. Values are expressed as means ± SE. B–D: hematoxylin-and-eosin histological classification of injury composite score. Animals were administered the following: Control-NaCl, HA (30 mg/kg), HA + SnMP (30 mg/kg HA and 40 μmol/kg SnMP), and SnMP (40 μmol/kg) alone by intravenous injection. After 24 h, these animals underwent the TRAM procedure. Methyl Carnoy's solution-fixed, paraffin-embedded sections were harvested 48 h after the animals underwent sham surgery (n = 4) (B), ischemia reperfusion injury (IRI)-Zone IV (n = 10) (C), and IRI- Zone I (n = 10) (D). Results were analyzed by one-way ANOVA and revealed significant differences between the treatment groups on this composite score outcome: [F(4,13) = 4.98, P = 0.0117] (B), [F(4, 33) = 21.9, P < 0.0001] (C), and [F(4,33) = 29.4, P < 0.0001] (D). The results of Tukey's post hoc test are denoted by *P < 0.05, **P < 0.01, and ***P < 0.001. Values are expressed as means ± SE.
HA Preconditioning Reduced Skin Perfusion
Assessment of flap perfusion, using laser-Doppler imaging, showed that preconditioning treatments did not affect preoperative perfusion (Fig. 5). In the immediate postoperative period there was a significant reduction in perfusion in control animals. HA pretreatment resulted in more significant reduction in perfusion compared with control. These changes were most profound 24 h after IRI and before significant necrosis had occurred and persisted to the end of the experiment. HA plus SnMP had similar reductions in perfusion while SnMP alone had no deleterious effects (Fig. 5). Thus, HA treatment exacerbated skin necrosis and reduced perfusion, an effect that was independent of HO-1 activity.
Fig. 5.
Mean flap perfusion as assessed by laser-Doppler imaging for the four preconditioning treatments: preoperatively (preop), immediately postoperatively (postop), at 24 h, and 48 h after the TRAM procedure. A significant difference between the pretreatment groups on this outcome measure was found by one-way ANOVA at all of the postoperative time points: postop (P = 0.0011), 24 h (P = 0.0002), and 48 h (P = 0.0004). Tukey's post hoc comparison test showed a significantly reduced perfusion in flaps treated with HA or HA + SnMP compared with controls at 24 and 48 h as indicated by *P < 0.05, **P < 0.01, and ***P < 0.001; n = 10. Values are expressed as means ± SE.
HA Preconditioning Does not Affect Systemic Blood Pressure
As part of the investigation into the unexpected deleterious effect of HA preconditioning on viability of myocutaneous flaps, the effect of HA on systemic blood pressure was investigated. Unpaired t-test of the average systolic blood pressure of HA pretreated rats (142.0 ± 4.62 mmHg) vs. Control (136 ± 4.65 mmHg) showed that HA did not affect systolic blood pressure (P = 0.38), data not shown graphically.
HA Administration Is Cytotoxic In Vitro
The effect of HA on human keratinocytes in vitro was assessed. HEKa cells were cultured to confluency and exposed to HA and/or SnMP in the presence or absence of the iron chelating agent desferrioxamine (DF). HA reduced cell survival, and these effects were reversed by desferrioxamine (Fig. 6). Coadministration of SnMP and HA did not affect cytotoxicity (Fig. 6). This suggests that free heme mediates the cytotoxic effects of HA preconditioning and that it is independent of HO-1 upregulation. Neither SnMP nor DF per se had significant effects on cytotoxicity (Fig. 6).
Fig. 6.
Mean cell viability as assessed by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay of human epidermal keratinocytes (HEKa) cells following 24 h of culture in the 12 preconditioning treatments. For clarity, only the results of control, 10 μmol HA, 10 μmol HA + 5 μmol SnMP, 5 μmol SnMP, 10 μmol HA + 500 μmol desferrioxamine (DF), 10 μmol HA + 50 μmol DF + 5 μmol SnMP, and 500 μmol DF are shown. A significant difference between the pretreatment groups on this outcome measure was found by one-way ANOVA, P < 0.0001. The seminal results of Tukey's test post hoc multiple comparisons are denoted by ns for not significant, **P < 0.01, and ***P < 0.001; n = 4. Values are expressed as means ± SE.
Cell viability was also assessed by the Vialight plus kit, which determines cellular ATP levels. HA reduced intracellular ATP levels which, as with cell viability, was reversed by DF. Neither SnMP nor DF per se had significant effects on cytotoxicity in this assay (Fig. 7).
Fig. 7.
Mean cell viability as assessed by Vialight plus kit in HEKa cells following 24 h of culture in nine preconditioning treatments. A significant difference between the pretreatment groups on this outcome measure was found by one-way ANOVA, P < 0.0001. For simplicity, only the results of control, 10 μmol HA, 10 μmol HA + 5 μmol SnMP, 5 μmol SnMP, 10 μmol HA + 500 μmol DF, and 500 μmol DF are shown. The critical results from Tukey's post hoc comparison are denoted by ns for not significant, **P < 0.01, and ***P < 0.001; n = 3. Values are expressed as means ± SE. RLU, relative light units.
HA Administration Leads to Increased Intracellular ROS Concentration
The MTT and Vialight assays corroborated the thesis that an HO-1-independent mechanism involving free iron was the likely mechanism by which the heme-containing preconditioning treatments were mediating cytotoxicity in HEKa culture. Free heme can act as a Fenton reactor to produce ROS. This was further assessed in HEKa cells. Intracellular ROS concentration was then assessed using CMH2DCFDA, which enters live cells, is cleaved by intracellular esterases (preventing its egress from the cell) and in the presence of ROS, forms the fluorescent product DCF. The geometric mean fluorescence was determined by FACS and represents the concentration of intracellular ROS in the cells (Fig. 8). HA treatment increased ROS generation in HEKa cells, and this was not affected by SnMP coadministration but was reversed by DF. This indicates that the ROS increase seen following administration of HA is independent of HO-1 upregulation and dependent on free iron.
Fig. 8.
Geometric mean fluorescence, an output measure for intracellular reactive oxygen species (ROS) concentration, was measured by FACS following treatment with CMH2DCFDA. The 12 preconditioning treatments were found to produce significant effects on intracellular ROS concentration by one-way ANOVA, P < 0.0001. For comprehensibility, the results of control, 10 μmol HA, 10 μmol HA + 5 μmol SnMP, 5 μmol SnMP, 10 μmol HA + 500 μmol DF, and 500 μmol DF are shown. The results of Tukey's post hoc comparison are denoted by **P < 0.01, ***P < 0.001, ns, not significant; n = 3. Values are expressed as means ± SE.
HA Administration Leads to Increased Oxidative Stress-Specific DNA Damage
To assess whether this increase in intracellular ROS following HA preconditioning (Fig. 8) caused measurable DNA damage and to quantify this, an 8-OHdg ELISA was performed (Fig. 9). HA preconditioning led to increased levels of 8-OHdg than controls, although this was not found to be statistically significant. HA + SnMP pretreatment led to the highest levels of 8-OHdg, and this was found to be significantly greater than control levels, P = 0.0003. These increased levels of 8-OHdg observed following HA/HA + SnMP pretreatment were completely reversed by coadministration of DF with these agents. Therefore, ROS generated from free heme is the most likely mediator of the increased injury seen following IRI in HA/HA and SnMP preconditioned myocutaneous flaps.
Fig. 9.
8-Hydroxy-2′-deoxguanosine (8-OHdg), a measure for oxidative stress-specific DNA damage. The 12 preconditioning treatments were found to produce significant effects on 8-OHdg levels by one-way ANOVA, P < 0.0001. The results of Tukey's post hoc comparison are denoted by **P < 0.01 and ***P < 0.001; n = 3. Values are expressed as means ± SE.
DISCUSSION
In agreement with other studies (41, 53, 62, 81), we have demonstrated that systemic administration of HA is effective in inducing HO-1 in the rat. The dose of 30 mg/kg was chosen on the basis of both earlier experimental work (Fig. 1) in our laboratory and published articles in the field, which showed this to be an effective dose at increasing HO-1 protein expression in the target tissues (40, 41, 48, 81). We had originally hypothesized that upregulation of HO-1 by HA would protect myocutaneous flaps against IRI, resulting in improved clinical outcome. However, HA treatment resulted in increased skin necrosis and reduced perfusion. These effects were independent of HO-1 activity. In vitro experiments demonstrated that HA reduced cell viability and induced ROS formation. These adverse effects were reversed by iron chelation with desferrioxamine and were not altered by HO-1 inhibition.
Previous studies have shown that HA can ameliorate IRI by upregulating HO-1 (3, 27, 47, 53, 106). The beneficial effects of HA in these models appears to be, at least in part, due to HA causing upregulation of HO-1 in macrophages. Systemic administration of HA to healthy volunteers upregulated HO-1 in plasma (6) and peripheral blood monocytes (20). In a murine model of kidney IRI, systemic upregulation of HO-1 by HA induced HO-1 in macrophages (27). Furthermore, chemical depletion of macrophages in this model abrogated the beneficial effects of HA (27). In more tissue-specific models, HO-1 upregulation, by heat/cooling, had successfully improved outcome in skin (37, 52), myocutaneous (77), and osteomyocutaneous flaps (78). In these tissues, heat preconditioning appeared to increase HO-1 in endothelial cells and pericytes, although this was not confirmed by immunohistochemistry (76) and in cells scattered between muscle fibers or within the subcutis (37, 52, 54). Of note, colocalization of HO-1 with specific cell markers to clarify in which cells HO-1 had been upregulated was not reported in these papers. The location and morphology of these HO-1-positive cells are consistent with them being macrophages. HO-1-expressing macrophages are known to be critical in cutaneous wound healing (34, 44, 104). Our study also demonstrated HO-1 upregulation in macrophages following HA administration. However, despite upregulating HO-1 in macrophages within the flap, preconditioning with HA led to significantly worse clinical outcome in this model.
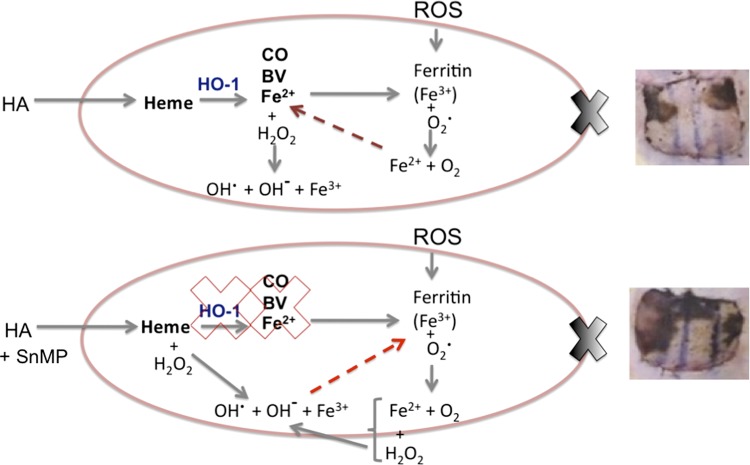
Free heme, i.e., intracellular heme not complexed to protein, is lipophilic and can act as a Fenton reactor to produce highly toxic hydroxyl radicals as can the ferrous iron (Fe2+) ions produced following degradation of free heme by HO-1 (26). As such, they can cause lipid peroxidation (80) and lead to cell membrane damage and apoptosis (5). Intracellular concentrations of free heme are consequently tightly regulated (82). It is unclear from our data whether it is free heme from the HA or ferrous iron ions from the degradation of that free heme by HO-1 is directly responsible for the production of ROS (Fig. 10). Ferritin is an antioxidant whose action is primarily mediated through the sequestration of reactive ferrous ions (Fe2+) and its reduction to ferric iron (Fe3+) by intrinsic ferroxidase activity (2, 110). However, under conditions of oxidative stress, ferritin releases its iron load, further exacerbating the oxidative stress by permitting Fe3+ to participate in oxidative reactions [Fig. 10 (73, 75, 118)]. Suttner and Dennery (92) showed that fibroblasts expressing high levels of HO-1 had high levels of ferritin protein, and this was associated with heightened oxygen toxicity. Despite not reaching statistical significance, a higher percentage area flap necrosis was seen (85% ± 3.6%) when HA was administered and HO-1 was inhibited (HA + SnMP preconditioned groups) than in the subjects treated with HA alone (69.7% ± 8.8%), as shown in Fig. 2. This is unsurprising, as by inhibiting HO-1, the cells' antioxidant capacity is further reduced by preventing the production of BV; see Fig. 10, lower image. Unfortunately, there is no way to confirm the location of these ferrous iron ions in the skin sections since these ferrous iron ions do not show up on Prussian blue staining (70).
Fig. 10.
Proposed mechanism of HA-mediated cell damage in the in vivo model. Top: upon administration of HA excess free heme results in the upregulation of HO-1 and production of CO, biliverdin (BV), and Fe2+. Both the free heme and Fe2+ can act as Fenton reactors to produce ROS. Fe2+ results in the expedient production of ferritin, which sequesters the reactive Fe2+ and oxidizes it to less reactive Fe3+. BV is rapidly converted by cytosolic biliverdin reductase to bilirubin, and both have powerful antioxidant activities to counteract the production of ROS from free heme and Fe2+. The scenario is complicated in the IRI model by the generation of ROS from reperfusion of previously ischemic tissues. This further strains the cells' intrinsic antioxidant capacity and may lead to the release of Fe3+ from ferritin, further exacerbating the production of ROS. This leads to necrosis (image, top, right). In the HA + SnMP-treated animals (bottom), the situation is worsened by the inhibition of HO-1. This further decreases the cells' ability to quench the ROS produced from the heme load from administration of HA and the IRI. This results in more extensive tissue damage (image, bottom, right). HA, heme arginate; HO-1, heme-oxygenase-1; CO, carbon monoxide; BV, biliverdin; Fe2+, ferrous iron; Fe3+, ferric iron; H2O2, hydrogen peroxide; OH−, hydroxyl ion; OH·, hydroxyl radical; ROS, reactive oxygen species; O2, oxygen; SnMP, tin mesoporphyrin.
Ferroportin is the only known cellular iron exporter in mammals and is present on hepatocytes, duodenal enterocytes, and other cells directly involved in the absorption and excretion of excess iron (30, 31). Neither this exporter nor any other divalent cation exporter has been described in human keratinocytes. The primary mechanism for removal of iron from the skin is desquamation of the epithelium (111). This may explain why HA preconditioning of the myocutaneous flaps identified in the composite injury score (Fig. 4) led to epithelial desquamation. Also, having abundant polyunsaturated fatty acids makes the skin particularly susceptible to ROS damage (98). Taken together, these findings suggest that the skin is more sensitive to iron-mediated cell damage than other organs, which may explain why a paradoxical worse injury was found compared with other experimental organ systems (27, 47, 53). Alternatively, the skin may be refractory to the predicted cytoprotective effects of HO-1 up-regulation as baseline levels of HO-1 are high in the skin, primarily within basal layer keratinocytes and macrophages (33, 34, 44, 104). The skin is constantly exposed to environmental stressors such as UV radiation, so it is unsurprising that there is high-baseline HO-1 expression (99). In this study, we showed HO-1 upregulation in the skin from baseline values, although this was less marked compared with other organs. In healthy humans treatment with 3 mg/kg HA produced a 75-fold (at 1 h) and 25-fold (at 24 h) increase in plasma heme concentration. It is probable that this would have been higher in our animal model given the larger dose administered (30 mg/kg). Despite inducing HO-1, such an increase in heme concentration may have exceeded HO-1's degradation capacity. This free heme not only produces ROS (26) but also activates NF-κB, AP-1, and AP-2 response elements, which regulate a number of proinflammatory genes, including E-selectin, ICAM-1, and vascular adhesion molecule 1 (45, 55). In iron overload disorders, such as hemochromatosis, there are prominent cutaneous manifestations with widespread skin dysfunction, indicating that the skin is sensitive to iron overload (16).
Perspectives and Significance
Other pharmacological agents that have been used to upregulate HO-1 successfully in animal models include sodium nitroprusside (102), cobalt (III) protoporphyrin (57), flavonoid 7-O-galloyltaxifolin (103), tin chloride (96), and quercetin (42). Unfortunately, these agents are not as readily translatable into clinical practice as HA would have been, as they do not have a similar profiles to HA regarding their use in humans. An alternative strategy to upregulate HO-1 is ischemic preconditioning (IPC) where multiple short periods of planned ischemia in the target tissue, which preconditions that tissue to better withstand a future more prolonged ischemic period and subsequent reperfusion. This has been used successfully in free flap models to reduce IRI (64, 85, 105). HO-1 has been proven to be fundamental for the protection afforded by IPC in animal models of muscle (22) and brain IRI (116). Local heat treatment to induce HO-1 as a preconditioning strategy could not be used in humans as the heat shock response element on the human HMOX-1 is nonfunctional (68, 114).
Pharmacological preconditioning with HA upregulated HO-1 in the skin in macrophages and other interstitial cells. Unlike solid organ IRI, the clinical outcome was worse due to augmented oxidative stress induced by free heme. HO-1-based therapies deserve further interrogation for their use as preconditioning strategies against myocutaneous IRI, although care should be taken when employing heme-based therapies in this tissue.
GRANTS
M.-C. Edmunds received the Medical Research Council Clinical Research Fellowship G1000299, Honorary Specialist Registrar National Health Service Lothian, Edinburgh, UK.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.-C.E., A.C., S.J.W., and D.C.K. conception and design of research; M.-C.E. and D.C.K. performed experiments; M.-C.E. analyzed data; M.-C.E. and D.C.K. interpreted results of experiments; M.-C.E. prepared figures; M.-C.E. and D.C.K. drafted manuscript; M.-C.E., S.J.W., and D.C.K. edited and revised manuscript; M.-C.E., A.C., S.J.W., and D.C.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank G. Blackie for his assistance with the images for this work, G. Borthwick for his assistance with the animal work, Dr. J. Baily for his assistance in devising the histological classification of injury and Drs. B. A. and G. M. Haddock for proofreading this manuscript.
REFERENCES
- 1.Andreas M, Schmid AI, Doberer D, Schewzow K, Weisshaar S, Heinze G, Bilban M, Moser E, Wolzt M. Heme arginate improves reperfusion patterns after ischemia: a randomized, placebo-controlled trial in healthy male subjects. J Cardiovasc Magn Reson 14: 55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 1790: 589–599, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Attuwaybi BO, Kozar RA, Moore-Olufemi SD, Sato N, Hassoun HT, Weisbrodt NW, Moore FA. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J Surg Res 118: 53–57, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J 19: 1216–1219, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Balla J, Vercellotti GM, KN, Yachie A, Nagy E, Eaton JW, Balla G. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transpl 18: v8–v12, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther 87: 187–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon AC, Hawkins CL, Bisht K, Coombes JS, Bakrania B, Wagner KH, Bulmer AC. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic Biol Med 52: 2120–2127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouard S, Otterbein LO, Anratherc J, Tobiascha E, Bach FH, Choib AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015–1026, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulmer AC, Blanchfield JT, Toth I, Fassett RG, Coombes JS. Improved resistance to serum oxidation in Gilbert's syndrome: a mechanism for cardiovascular protection. Atherosclerosis 199: 390–396, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bulmer AC, Verkade HJ, Wagner KH. Bilirubin and beyond: A review of lipid status in Gilbert's syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res 52: 193–205, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Burger D, Xiang F, Hammoud L, Lu X, Feng Q. Role of heme oxygenase-1 in the cardioprotective effects of erythropoietin during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 296: H84–H93, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 250–266, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Carmo-Araujo EM, Dal-Pai-Silva M, Dal-Pai V, Cecchini R, Anjos Ferreira AL. Ischaemia and reperfusion effects on skeletal muscle tissue: morphological and histochemical studies. Int J Exp Pathol 88: 147–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA 102: 7251–7256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Chevrant-Breton J, Simon M, Bourel M, Ferrand B. Cutaneous manifestations of idiopathic hemochromatosis. Study of 100 cases. Arch Dermatol 113: 161–165, 1977 [PubMed] [Google Scholar]
- 17.Condren AB, Kumar A, Mettu P, Liang KL, Zhao L, Tsai JY, Fariss RN, Wong WT. Perivascular mural cells of the mouse choroid demonstrate morphological diversity that is correlated to vasoregulatory function. PLos ONE 8: e53386, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods 160: 81–88, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Devey L, Mohr E, Bellamy C, Simpson K, Henderson N, Harrison EM, Ross JA, Wigmore SJ. c-Jun terminal kinase-2 gene deleted mice overexpress hemeoxygenase-1 and are protected from hepatic ischemia reperfusion injury. Transplantation 88: 308–316, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Doberer D, Haschemi A, Andreas M, Zapf T, Clive Jeitler M, Heinzl H, Wagner O, Wolzt M, Bilban M. Haem arginate infusion stimulates haem oxygenase-1 expression in healthy subjects. Br J Pharmacol 161: 1751–1762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duckers HJ, Boehm M, ALT, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 7: 693–698, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Dungey AA, Badhwar A, Bihari A, Kvietys PR, Harris KA, Forbes TL, Potter RF. Role of heme oxygenase in the protection afforded skeletal muscle during ischemic tolerance. Microcirculation 13: 71–79, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Edmunds MC, Wigmore S, Kluth D. In situ transverse rectus abdominis myocutaneous flap: a rat model of myocutaneous ischemia reperfusion injury. J Vis Exp 10.3791/50473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Brit Med Bull 70: 71–86, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594: 57–72, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Fenton HJH. LXXIII. Oxidation of tartaric acid in presence of iron. J Chem Soc 65: 899–910, 1894 [Google Scholar]
- 27.Ferenbach DA, Nkejabega NC, McKay J, Choudhary AK, Vernon MA, Beesley MF, Clay S, Conway BC, Marson LP, Kluth DC, Hughes J. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int 79: 966–976, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Ferenbach DA, Ramdas V, Spencer N, Marson L, Anegon I, Hughes J, Kluth DC. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol Ther 18: 1706–1713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukui A, Inada Y, Murata K, Tamai S. Plasmatic imbibition in the rabbit flow-through venous flap, using horseradish peroxidase and fluoroscein. J Reconstr Microsurg 11: 255–264, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Garrick MD. Human iron transporters. Genes Nutr 6: 45–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrick MD, Garrick LM. Cellular iron transport. Biochim Biophys Acta 1790: 309–325, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Grochot-Przeczek A, Lach R, Mis J, Skrzypek K, Gozdecka M, Sroczynska P, Dubiel M, Rutkowski A, Kozakowska M, Zagorska A, Walczynski A, Was H, Kotlinowski J, Drukala J, Kurowski K, Kieda C, Herault Y, Dulak J, Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLos ONE 4: e5803, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanselmann C, Mauch C, Werner S. Haem oxygenase-1: a novel player in cutaneous wound repair and psoriasis? Biochem J 353: 459–466, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harder Y, Amon M, Schramm R, Rucker M, Scheuer C, Pittet B, Erni D, Menger MD. Ischemia-induced up-regulation of heme oxygenase-1 protects from apoptotic cell death and tissue necrosis. J Surg Res 150: 293–303, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Harder Y, Amon M, Laschke MW, Schramm R, Rucker M, Wettstein R, Bastiaanse J, Frick A, Machens HG, Kuntscher M, Germann G, Vollmar B, Erni D, Menger MD. An old dream revitalised: preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg 61: 503–511, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Harder Y, Amon M, Schramm R, Georgi M, Banic A, Erni D, Menger MD. Heat shock preconditioning reduces ischemic tissue necrosis by heat shock protein (HSP)-32-mediated improvement of the microcirculation rather than induction of ischemic tolerance. Ann Surg 242: 869–878, discussion 878–869, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hira S, Tomita T, Matsui T, Igarashi K, Ikedo-Saito M. Bach1, a heme-dependent transcription factor, reveals presence of multiple heme binding sites with distinct coordination structure. IUBMB Life 59: 542–551, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal 8: 107–118, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Jadhav A, Ndisang JF. Heme arginate suppresses cardiac lesions and hypertrophy in deoxycorticosterone acetate-salt hypertension. Exp Biol Med (Maywood) 234: 764–778, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Jadhav A, Ndisang JF. Treatment with heme arginate alleviates adipose tissue inflammation and improves insulin sensitivity and glucose metabolism in a rat model of human primary aldosteronism. Free Radic Biol Med 53: 2277–2286, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Jie Q, Tang Y, Deng Y, Li Y, Shi Y, Gao C, Xing M, Wang D, Liu L, Yao P. Bilirubin participates in protecting of heme oxygenase-1 induction by quercetin against ethanol hepatotoxicity in cultured rat hepatocytes. Alcohol 47: 141–148, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Juniantito V, Izawa T, Yuasa T, Ichikawa C, Yamamoto E, Kuwamura M, Yamate J. Immunophenotypical analyses of myofibroblasts in rat excisional wound healing: possible transdifferentiation of blood vessel pericytes and perifollicular dermal sheath cells into myofibroblasts. Histol Histopathol 27: 515–527 [DOI] [PubMed] [Google Scholar]
- 44.Kampfer H, Kolb N, Manderscheid M, Wetzler C, Pfeilschifter J, Frank S. Macrophage-derived heme-oxygenase-1: expression, regulation, and possible functions in skin repair. Mol Med 7: 488–498, 2001 [PMC free article] [PubMed] [Google Scholar]
- 45.Kanakiriya SKR, Croatt AJ, Haggard JJ, Ingelfinger JR, Tang SS, Alam J, Nath KA, Balla G, Juckett MB, Jacob HS, Vercellotti GM. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol Renal Physiol 284: F546–F554, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol 33: 125–130, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Eum HA, Billiar TR, Lee SM. Role of heme oxygenase 1 in TNF/TNF receptor-mediated apoptosis after hepatic ischemia/reperfusion in rats. Shock 39: 380–388, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Kim SJ, Eum HA, Billiar TR, Lee SM. Role of heme oxygenase 1 in TNF/TNF receptor-mediated apoptosis after hepatic ischemia/reperfusion in rats. Shock 39: 380–388, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Kinderlerer ARGI, Hambuley SS, ALi F, Steinberg R, Silva G, Ali N, Wang B, Haskard DO, Soares MP, Mason JC. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin. Blood 113: 1598–1606, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Koizumi S. Human heme oxygenase-1 deficiency: a lesson on serendipity in the discovery of the novel disease. Pediatr Int 49: 125–132, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Kreutz R, Kovacevic L, Schulz A, Rothermund L, Ketteler M, Paul M. Effect of high NaCl diet on spontaneous hypertension in a genetic rat model with reduced nephron number. J Hypertens 18: 777–782, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Kubulus D, Amon M, Roesken F, Rucker M, Bauer I, Menger MD. Experimental cooling-induced preconditioning attenuates skin flap failure. Br J Surg 92: 1432–1438, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Kubulus D, Mathes A, Pradarutti S, Raddatz A, Heiser J, Pavlidis D, Wolf B, Bauer I, Rensing H. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock 29: 583–590, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Kubulus D, Roesken F, Amon M, Rucker M, Bauer M, Bauer I, Menger MD. Mechanism of the delay phenomenon: tissue protection is mediated by heme oxygenase-1. Am J Physiol Heart Circ Physiol 287: H2332–H2340, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-κB and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA 91: 5987–5991, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee C, Kerrigan CL, Tellado JM. Altered neutrophil function following reperfusion of an ischemic myocutaneous flap. Plast Reconstr Surg 89: 916–923, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Lee IT, Luo SF, Lee CW, Wang SW, Lin CC, Chang CC, Chen YL, Chau LY, Yang CM. Overexpression of HO-1 protects against TNF-α-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am J Pathol 175: 519–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SF, Pervaiz S. Assessment of oxidative stress-induced DNA damage by immunoflourescent analysis of 8-OxodG. In: Methods in Cell Biology, edited by Darzynkiewicz Z. Santa Barbara, CA: Academic, 2011, chap. 5, p. 99–113 [DOI] [PubMed] [Google Scholar]
- 59.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8: 240–246, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler Thromb Vasc Biol 24: 601–606, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Lo SS, Lin SC, Wu CW, Chen JH, Yeh WI, Chung MY, Lui WY. Heme oxygenase-1 gene promoter polymorphism is associated with risk of gastric adenocarcinoma and lymphovascular tumor invasion. Ann Surg Oncol 14: 2250–2256, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Maeshima K, Takahashi T, Uehara K, Shimizu H, Omori E, Yokoyama M, Tani T, Akagi R, Morita K. Prevention of hemorrhagic shock-induced lung injury by heme arginate treatment in rats. Biochem Pharmacol 69: 1667–1680, 2005 [DOI] [PubMed] [Google Scholar]
- 63.McNally SJ, Ross JA, Garden OJ, Wigmore SJ. Optimization of the paired enzyme assay for heme oxygenase activity. Anal Biochem 332: 398–400, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, Zair M, Rassuli A, Forrest CR, Grover GJ, Pang CY. Mitochondrial KATP channels in hindlimb remote ischemic preconditioning of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol 288: H559–H567, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Nys K, Maes H, Andrei G, Snoeck R, Garmyn M, Agostinis P. Skin mild hypoxia enhances killing of UVB-damaged keratinocytes through reactive oxygen species-mediated apoptosis requiring Noxa and Bim. Free Radic Biol Med 52: 1111–1120, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J 20: 2835–2843, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ojima A, Ishibashi Y, Matsui T, Maeda S, Nishino Y, Takeuchi M, Fukami K, Yamagishi SI. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol 182: 132–141, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Okinaga S, Takahasi KM, Takeda K, Yoshizawa M, Fujita H, Sasaki H, Shibahara S. Regulation of human heme-oxygenase-1 gene expression under thermal stress. Blood 87: 5074–5084, 1996 [PubMed] [Google Scholar]
- 69.Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, Choi AMK. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Perls M. Nachweis von eisenoxyl in gewissen pigmenten. Virchows Arch Path Anat 39: 42–48, 1867 [Google Scholar]
- 71.Picard-Ami LA, MacKay A, Kerrigan CL. Effect of allopurinol on the survival of experimental pig flaps. Plast Reconstr Surg 89: 1098–1103, 1992 [DOI] [PubMed] [Google Scholar]
- 72.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA 94: 10925–10930, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pourzand C, Watkin RD, Brown JE, Tyrrell RM. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: The role of ferritin. Proc Natl Acad Sci USA 96: 6751–6756, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radhakrishnan N, Yadav S, P, Sachdeva A, Pruthi PK, Sawhney S, Piplani T, Wada T, Yachie A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol 33: 74–78, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Reif DW. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med 12: 417–427, 1992 [DOI] [PubMed] [Google Scholar]
- 76.Rucker M, Schafer T, Roesken F, Spitzer WJ, Bauer M, Menger MD. Reduction of inflammatory response in composite flap transfer by local stress conditioning-induced heat-shock protein 32. Surgery 129: 292–301, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Rucker M, Schafer T, Roesken F, Spitzer W, Bauer M, Menger M. Reduction of inflammatory response in composite flap transfer by local stress conditioning-induced heat-shock protein 32. Surgery 129: 292–301, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Rucker M, Schafer T, Roesken F, Spitzer WJ, Bauer M, Menger MD. Local heat-shock priming-induced improvement in microvascular perfusion in osteomyocutaneous flaps is mediated by heat-shock protein 32. Brit J Surg 88: 450–457, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Rucker M, Schafer T, Stamm A, Saueressig K, Vollmar B, Spitzer WJ, Menger MD. New model for in vivo quantification of microvascular embolization, thrombus formation, and recanalization in composite flaps. J Surg Res 108: 129–137, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28: 289–309, 2000 [DOI] [PubMed] [Google Scholar]
- 81.Sasaki T, Takahashi T, Maeshima K, Shimizu H, Toda Y, Morimatsu H, Takeuchi M, Yokoyama M, Akagi R, Morita K. Heme arginate pretreatment attenuates pulmonary NF-κB and AP-1 activation induced by hemorrhagic shock via heme oxygenase-1 induction. Med Chem 2: 271–274, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Sassa S. Why heme needs to be degraded to iron, biliverdin IXα, and carbon monoxide? Antioxid Redox Sign 6: 819–824, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, Geiger KK, Pannen BH. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg 245: 931–942, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah AA, Arias JE, Thomson JG. The effect of ischemic preconditioning on secondary ischemia in myocutaneous flaps. J Reconstr Mirosurg 25: 527–531, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Sharma V, Ling TW, Rewell SS, Hare DL, Howells DW, Kourakis A, Wookey PJ. A novel population of alpha-smooth muscle actin-positive cells activated in a rat model of stroke: an analysis of the spatio-temporal distribution in response to ischemia. J Cereb Blood Flow Metab 32: 2055–2065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siemonow M, Arslan E. Ischaemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery 24: 468–475, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Soares MP, Bach FH. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med 15: 50–58, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 90.Stotland MJ, Kerrigan CL. The principle of skin flap surgery. In: Plastic Surgery Secrets Plus, edited by Weinzweig J. Philadelphia, PA: Mosby Elsevier, 2010, p. 684–687 [Google Scholar]
- 91.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 21: 5216–5224, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 13: 1800–1809, 1999 [DOI] [PubMed] [Google Scholar]
- 93.Suzuki H, Tashiro S, Hira S, Sun J, Yamazaki C, Zenke Y, Ikeda-Saito M, Yoshida M, Igarashi K. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J 23: 2544–2553, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tindholdt TT, Saidian S, Pripp AH, Tonseth KA. Monitoring microcirculatory changes in the deep inferior epigastric artery perforator flap with laser Doppler perfusion imaging. Ann Plast Surg 67: 139–142, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Tindholdt TT, Saidian S, Tonseth KA. Microcirculatory evaluation of deep inferior epigastric artery perforator flaps with laser Doppler perfusion imaging in breast reconstruction. J Plast Surg Hand Surg 45: 143–147, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Toda N, Takahashi T, Mizobuchi S, Fujii H, Nakahira K, Takahashi S, Yamashita M, Morita K, Hirakawa M, Akagi R. Tin chloride pretreatment prevents renal injury in rats with ischemic acute renal failure. Crit Care Med 30: 1512–1522, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Katusic ZS, Nath KA. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol 173: 1882–1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trenam CW, Blake DR, Morris CJ. Skin inflammation: reactive oxygen species and the role of iron. J Invest Dermatol 99: 675–682, 1992 [DOI] [PubMed] [Google Scholar]
- 99.Tyrrell R. Redox regulation and oxidant activation of heme oxygenase-1. Free Radic Res 31: 335–340, 1999 [DOI] [PubMed] [Google Scholar]
- 100.van den Heuvel MG, Buurman WA, Bast A, van der Hulst RR. Ischaemia-reperfusion injury in flap surgery. J Plast Reconstr Aesthet Surg 62: 721–726, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Veerappan A, Reid AC, Estephan R, O'Connor N, Thadani-Mulero M, Salazar-Rodriguez M, Levi R, Silver RB. Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci USA 105: 1315–1320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vesely MJ, Exon DJ, Clark JE, Foresti R, Green CJ, Motterlini R. Heme oxygenase-1 induction in skeletal muscle cells: hemin and sodium nitroprusside are regulators in vitro. Am J Physiol Cell Physiol 275: C1087–C1094, 1998 [DOI] [PubMed] [Google Scholar]
- 103.Vrba J, Gazak R, Kuzma M, Papouskova B, Vacek J, Weiszenstein M, Kren V, Ulrichova J. A novel semisynthetic flavonoid 7-O-galloyltaxifolin upregulates heme oxygenase-1 in RAW264.7 cells via MAPK/Nrf2 pathway. J Med Chem 56: 856–866, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Wagener FA, van Beurden HE, von den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system: a molecular switch in wound healing. Blood 102: 521–528, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Wang H, Zhiyong L, Xiolin L. Effects of various protocols of ischemic preconditioning on rat TRAM flaps. Microsurgery 28: 37–43, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Wang W, Wang F, Shi L, Jia X, Lin L. Role of heme oxygenase-1/carbon monoxide system in pulmonary ischemia-reperfusion injury. Interact Cardiovasc Thorac Surg 9: 159–162, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Wang WZ. Investigation of reperfusion injury and ischemic preconditioning in microsurgery. Microsurgery 29: 72–79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg 128: 685e–692e, 2011 [DOI] [PubMed] [Google Scholar]
- 109.Wang WZ, Fang XH, Stephenson LL, Zhang X, Khiabani KT, Zamboni WA. Melatonin attenuates I/R-induced mitochondrial dysfunction in skeletal muscle. J Surg Res 171: 108–113, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Watt RK. The many faces of the octahedral ferritin protein. Biometals 24: 489–500, 2011 [DOI] [PubMed] [Google Scholar]
- 111.Weintraub LR, Demis DJ, Conrad ME, Crosby WH. Iron excretion by the skin. Selective localization or iron-59 in epthelial cells. Am J Pathol 46: 121–127, 1965 [PMC free article] [PubMed] [Google Scholar]
- 112.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 66: 187–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yeh CH, Chen TP, Wang YC, Lin YM, Lin PJ. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-κB and AP-1 translocation following cardiac global ischemia and reperfusion. J Surg Res 155: 147–156, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Yoshida T, Biro P, Chen R, Muller RM, Shibara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem 171: 457–461, 1988 [DOI] [PubMed] [Google Scholar]
- 115.Zenke-Kawasaki Y, Dohi Y, Katk Y, Ikura M, Asahra T, Tokunaga F, Iwai K, Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol 27: 6962–6971, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeynalov E, Shah ZA, Li RC, Dore S. Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis 35: 264–269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem 279: 10677–10684, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Zhong JL, Yiakouvaki A, Holley P, Tyrrell RM, Pourzand C. Susceptibility of skin cells to UVA-induced necrotic cell death reflects the intracellular level of labile iron. J Invest Dermatol 123: 771–780, 2004 [DOI] [PubMed] [Google Scholar]