Abstract
CD20 is a B cell differentiation antigen with variable expression in B cell precursor acute lymphoblastic leukemia (BCP-ALL). The significance of CD20 expression has been evaluated in BCP-ALL with conflicting results. There is paucity of data regarding CD 20 expression in BCP-ALL in Indian patients. We retrospectively analyzed 100 patients of BCP-ALL for CD20 expression. CD20 positivity was defined as expression of CD20 to be more than or equal to 20 % in the blast population. 62 % patients expressed CD20 while 38 % patients were negative for CD20. The positivity ranged from negative to dim (35.5 % patients), moderately bright (19.3 % patients) to bright (45.2 % patients). Additional prospective studies are needed to determine the optimal use of rituximab in treatment of CD20-positive BCP-ALL.
Keywords: BCP-ALL, CD20 antigen, Rituximab
Introduction
In acute lymphoblastic leukemia (ALL), immunophenotype plays a crucial diagnostic role and is prognostically informative. CD20 is a B cell differentiation antigen with variable expression in B cell precursor acute lymphoblastic leukemia (BCP-ALL). Its expression has been associated with a better [1] or an inferior outcome in ALL [2], probably depending on the treatment used. CD20 expression ranges from 30 to 50 % in BCP-ALL compared with 80–90 % in mature B cell or Burkitt-type leukemia/lymphoma [3]. Data from childhood BCP-ALL suggests that induction chemotherapy frequently induces significant up-regulation of CD20 expression even if CD20 expression had been negative at the time of diagnosis [4].
As intensification of ALL chemotherapy is reaching a limit, targeted therapy may further improve treatment outcome by increasing the efficacy and decreasing the toxicity of standard regimens. ALL blast cells express a variety of antigens which may serve as targets, such as CD19, CD20, CD22, CD33, and CD52 [5]. In line with the expression patterns, anti-CD20 directed immunotherapy has been shown to elicit potent antitumor effects specifically in mature B cell lymphoma and leukemia, where it has been incorporated into standard treatment as a valuable therapy advance [6].To date, the most broadly evaluated compound for CD20 targeting is rituximab, a chimeric monoclonal antibody. In mature B-lineage (Burkitt-type) ALL the incorporation of rituximab has significantly improved the outcome [7]. Some studies have reported similar preliminary findings of improvements in outcome with rituximab-based chemoimmunotherapy for CD20-positive precursor B cell ALL [8, 9].
The present study is based on CD20 expression evaluated on 100 patients of BCP-ALL with a view to determine its potential for therapy using rituximab at a tertiary care center in north India.
Materials and Methods
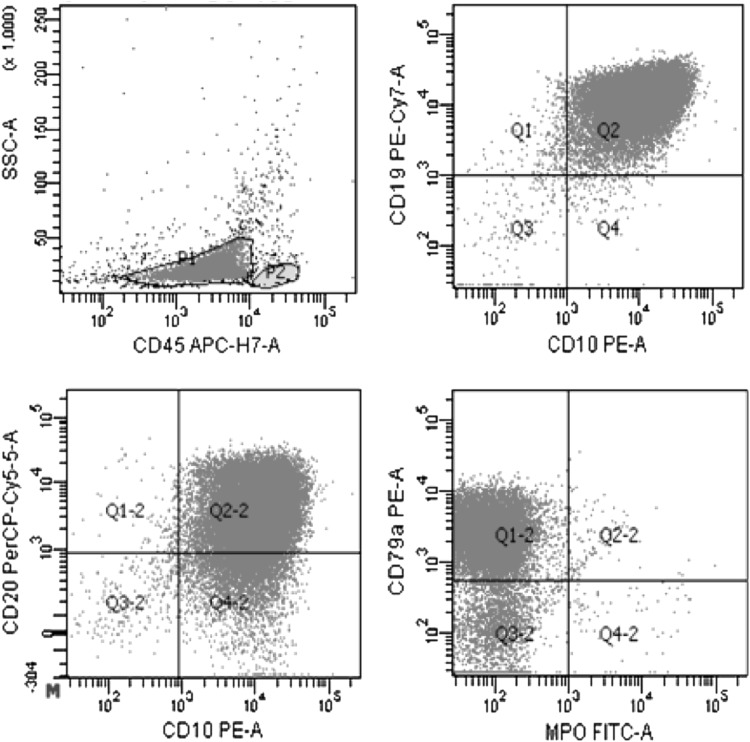
This study included 100 patients with BCP-ALL investigated at Sir Ganga Ram Hospital, New Delhi, India. CD20 positivity was defined as expression of CD20 to be more than or equal to 20 % in the blast population [4]. Immunophenotypic analyses were performed on RBC-lysed whole bone marrow aspirate and peripheral blood samples. Six-color flowcytometric immunophenotypic analysis was conducted using BDFACS CANTO-II (BD-Biosciences). Acute leukemia panel comprising of monoclonal antibodies cCD79a, CD19, CD10, CD20, cCD3, CD2, CD4, CD5, CD7, CD8, cMPO, CD33, CD117, CD14, CD64, CD34, TdT, CD58 and CD38 was used (Fig. 1).
Fig. 1.
Flow cytometric immunophenotyping in a CD20+ B cell precursor ALL patient CD45/SSC dot plot shows a blast cluster with low SSC and dim CD45. These cells express cCD79a, CD19, CD10 and CD20
Results
Age of patients ranged from 4 months to 65 years (Table 1). Out of 100 patients with BCP-ALL, 62 (62 %) expressed CD20 while 38 (38 %) were negative for CD20. The CD20 positivity ranged from dim (22 patients, 35.5 %) to bright (40 patients, 64.5 %). Difference in mean total leukocyte count (TLC) in CD20+ (25,701.7/μl) and CD20− (25,755.9/μl) cases was not significant (P > 0.05). Difference in mean peripheral blast count in CD20+ (52.9 %) and CD20− (53.5 %) cases was not significant (P > 0.05). Hepatomegaly (45.2 % in CD20+ cases vs. 28.9 % in CD− cases) and splenomegaly (43.5 % in CD20+ cases vs. 28.9 % in CD− cases) were more frequently seen in CD20+ cases.
Table 1.
Patients’ characteristics
| Total | CD20+ | CD20− | |
|---|---|---|---|
| No. of patients | 100 | 62 (62 %) | 38 (38 %) |
| Mean age | 9.2 (4 months–65 years) | 9.5 (1–65 Years) | 8.7 (4 months–45 years) |
| Sex | |||
| Male | 67 | 43 (64.2 %) | 24 (35.8 %) |
| Female | 33 | 19 (57.6 %) | 14 (42.4 %) |
| Mean Hb (in g/dl) | 7.61 | 7.49 | 7.8 |
| Mean TLC (per μl) | 25,721.5 (400–2,21,700) | 25,701.7 (400–2,21,700) | 25,755.9 (1,500–1,40,800) |
| Mean platelets count (per μl) | 64,270 | 59,451.6 | 72,131.6 |
| Mean peripheral blasts (%) | 53.1 (0–96) | 52.9 (0–96) | 53.5 (0–96) |
| Splenomegaly | 38 (38 %) | 27 (43.5 %) | 11 (28.9 %) |
| Hepatomegaly | 39 (39 %) | 28 (45.2 %) | 11 (28.9 %) |
Discussion
There is paucity of data regarding CD 20 expression in BCP-ALL in Indian patients. This study shows that a total of 62 % patients of BCP-ALL in all age groups expressed CD20, which is higher than the figures reported from western world (30–50 %). So anti-CD20 directed immunotherapy like rituximab can significantly improve the outcome in precursor B cell ALL in this population. This data can be deployed for further studies with a view for therapeutic intervention. Additional prospective studies are needed to determine the optimal use of rituximab in treatment of CD20-positive (and possibly CD20 negative subset based on up-regulation of CD20 expression) BCP-ALL.
References
- 1.Jeha S, Behm F, Pei D, et al. Prognostic significance of CD20 expression in childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2006;108:3302–3304. doi: 10.1182/blood-2006-04-016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowitz MJ, Shuster J, Carroll AJ, et al. Prognostic significance of fluorescence intensity of surface marker expression in childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 1997;89:3960–3966. [PubMed] [Google Scholar]
- 3.Gökbuget N, Hoelzer D. Treatment with monoclonal antibodies in acute lymphoblastic leukemia: current knowledge and future prospects. Ann Hematol. 2004;83(4):201–205. doi: 10.1007/s00277-003-0752-8. [DOI] [PubMed] [Google Scholar]
- 4.Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982–3988. doi: 10.1182/blood-2008-06-164129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maury S, Huguet F, Leguay T, Lacombe F, Maynadié M, Girard S, de Labarthe A, Kuhlein E, Raffoux E, Thomas X, Chevallier P, Buzyn A, Delannoy A, Chalandon Y, Vernant J-P, Rousselot P, Macintyre E, Ifrah N, Dombret H, Béné MC, Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) Adverse prognostic significance of CD20 expression in adults with Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia. Haematologica. 2010;95:324–328. doi: 10.3324/haematol.2009.010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5:292–302. doi: 10.1016/S1470-2045(04)01467-6. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DA, Faderl S, O’Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus Rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 8.Claviez A, Eckert C, Seeger K, et al. Rituximab plus chemotherapy in children with relapsed or refractory CD20 B-cell precursor acute lymphoblastic leukemia. Haematologica. 2006;91:272–273. [PubMed] [Google Scholar]
- 9.Morris ES, Vora A. Remission induction with single agent Rituximab in a child with multiply relapsed precursor-B ALL. Br J Haemotol. 2007;139:344–345. doi: 10.1111/j.1365-2141.2007.06814.x. [DOI] [PubMed] [Google Scholar]