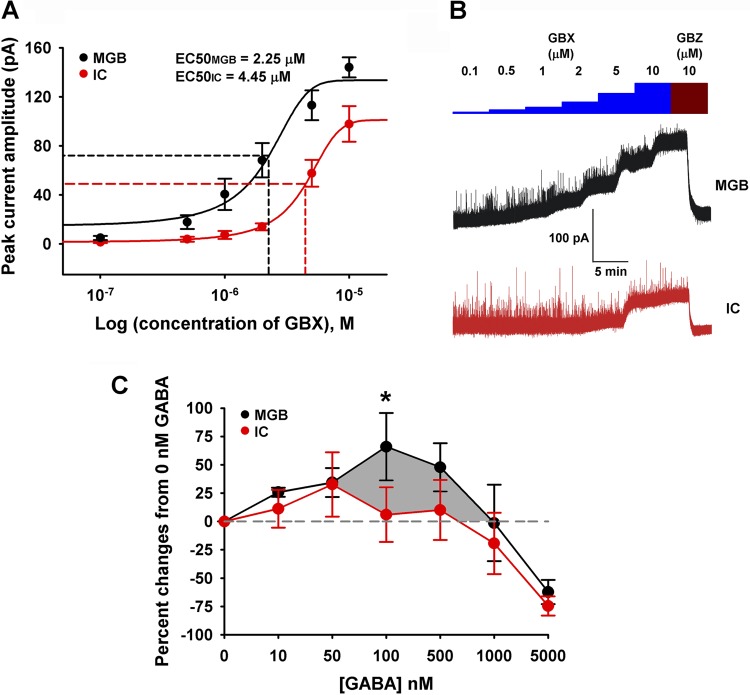
Fig. 3.
In vitro: GBX induced tonic inhibition and [3H]t-butylbicycloorthobenzoate ([3H]TBOB) binding in brain slices. Whole cell patch-clamp recordings from 5 IC and 5 MGB neurons from adult slices were used to compare the relative sensitivity of extrasynaptic GABAARs in IC and MGB neurons. A: tonic currents were plotted against increasing dosages of GBX. GBX was significantly more potent in activating tonic currents in MGB units than in IC units [EC50MGB = 2.25 μM; EC50IC = 4.45 μM; F (1, 54) = 30.17, P < 0.0001; F-test]. B: bath application of increasing doses (0.1–10 μM) of the δ-GABAAR subunit-selective agonist, GBX, evoked tonic currents in both IC and MGB neurons. Amplitudes of GBX-evoked tonic currents were revealed by addition of 10 μM gabazine (GBZ). C: modulation of [3H]TBOB channel (picrotoxin) binding with increasing concentrations of GABA (0 nM to 5 μM) was performed on IC and MGB slices. 0 nM GABA was set as the control condition and represented the resting/control openings of GABAAR Cl− channels. At low concentrations, both structures showed increased binding indicative of increased GABAAR, Cl− channels openings. Peak percent increase in binding occurred at 100 nM for MGB (*P < 0.05, 2-way ANOVA) and 50 nM for IC, with a significantly larger area (shadow) under the MGB curve (black) suggesting a greater MGB neuronal total chloride flux capacity relative to IC neurons. Both MGB and IC showed desensitization (1,000 nM and 5,000 nM) reflecting a greater percentage of closed GABAAR Cl− channels than in the control condition.