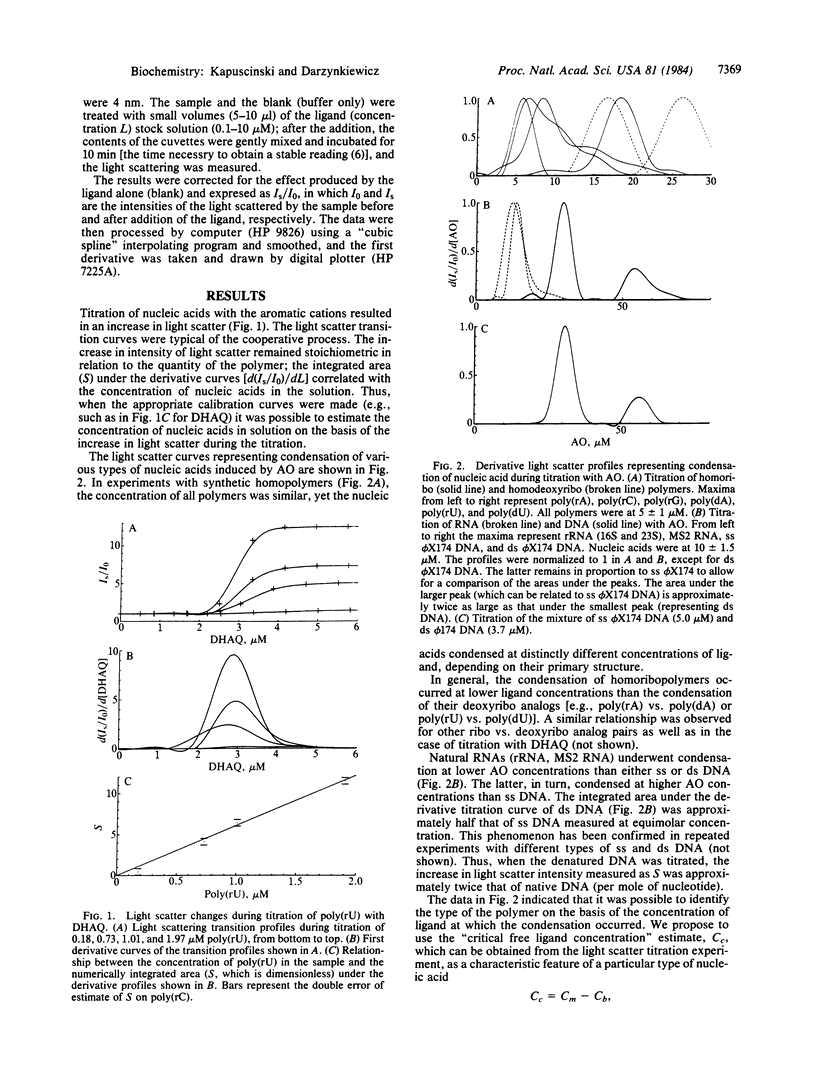
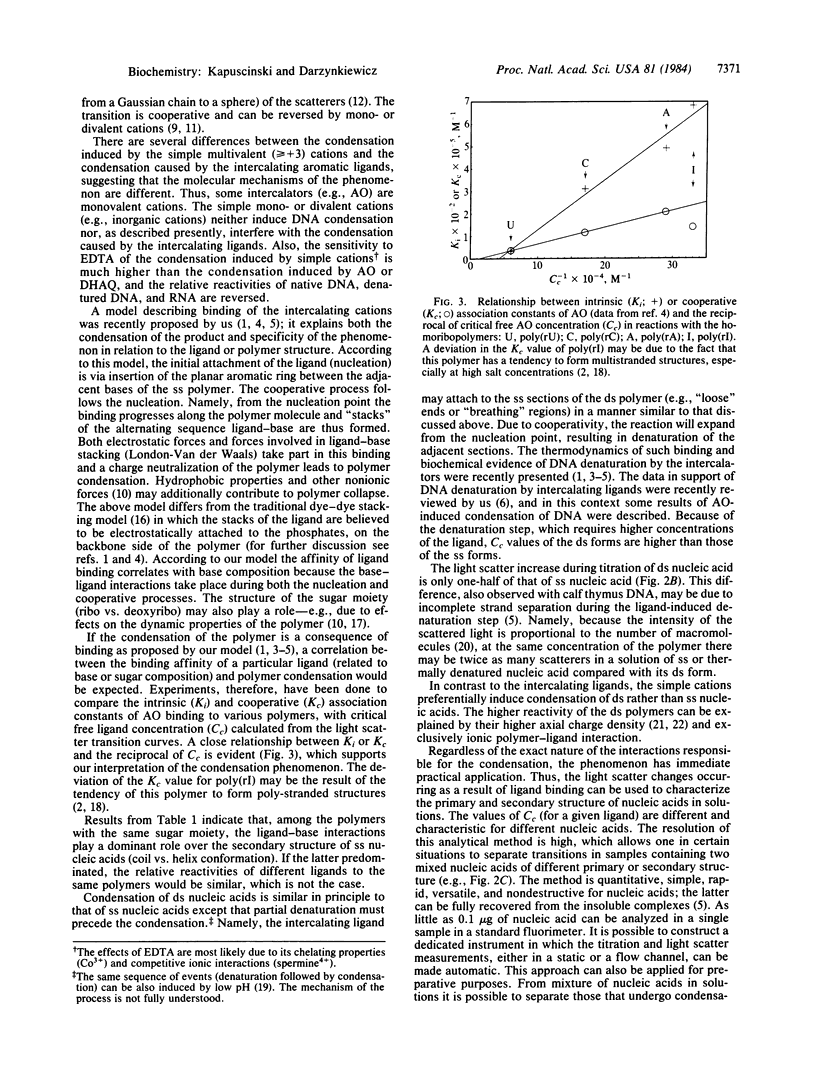
Abstract
Certain intercalating aromatic cations, such as the fluorochrome acridine orange or the antitumor drug Mitoxantrone, induce condensation of nucleic acids in solutions. The appearance of the condensed form during titration of nucleic acids with these intercalating ligands can be quantitatively monitored by light scatter measurements. The resulting highly reproducible light scatter transition curves are typical of the cooperative processes, and the transitions occur at different critical concentrations of the ligands depending upon both the ligand itself and the primary structure (base and sugar composition) and the secondary structure (single- or double-stranded) of the nucleic acids. The mechanism of condensation of nucleic acids by intercalating cationic ligands is discussed in light of the model of interactions occurring between certain intercalators and single-stranded nucleic acids and compared with the condensation induced by polyvalent "simple" cations such as Co3+ or spermine4+. The described phenomenon can have an application in analytical and preparative biochemistry for characterization of the primary and secondary structure of nucleic acids and for separation of the compounds. The possibility that the condensation plays a role in mutagenic and pharmacological effects of aromatic cations is considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. F., Wolf M. K. AGGREGATION OF DYES BOUND TO POLYANIONS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):944–952. doi: 10.1073/pnas.45.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. H., Thomas G. J., Jr, Arnott S., Smith P. J. Raman spectral studies of nucleic acids. XVII. Conformational structures of polyinosinic acid. Nucleic Acids Res. 1977 Jul;4(7):2407–2419. doi: 10.1093/nar/4.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D., Kapuscinski J., Melamed M. R. Denaturation of RNA and DNA in situ induced by acridine orange. Exp Cell Res. 1983 Oct;148(1):31–46. doi: 10.1016/0014-4827(83)90185-4. [DOI] [PubMed] [Google Scholar]
- Dore E., Frontali C., Gratton E. Physico-chemical description of a condensed form of DNA. Biopolymers. 1972 Feb;11(2):443–459. doi: 10.1002/bip.1972.360110210. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Denaturation of nucleic acids induced by intercalating agents. Biochemical and biophysical properties of acridine orange-DNA complexes. J Biomol Struct Dyn. 1984 Jun;1(6):1485–1499. doi: 10.1080/07391102.1984.10507532. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Increased accessibility of bases in DNA upon binding of acridine orange. Nucleic Acids Res. 1983 Nov 11;11(21):7555–7568. doi: 10.1093/nar/11.21.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Melamed M. R. Interactions of acridine orange with nucleic acids. Properties of complexes of acridine orange with single stranded ribonucleic acid. Biochem Pharmacol. 1983 Dec 15;32(24):3679–3694. doi: 10.1016/0006-2952(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Melamed M. R. Luminescence of the solid complexes of acridine orange with RNA. Cytometry. 1982 Jan;2(4):201–211. doi: 10.1002/cyto.990020402. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Traganos F., Melamed M. R. Interactions of a new antitumor agent, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]-ethyl]amino]-9,10-anthracenedione, with nucleic acids. Biochem Pharmacol. 1981 Feb 1;30(3):231–240. doi: 10.1016/0006-2952(81)90083-6. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Locher S. E., Meyn R. E. Relationship between cytotoxicity and DNA damage in mammalian cells treated with anthracenedione derivatives. Chem Biol Interact. 1983 Sep 15;46(3):369–379. doi: 10.1016/0009-2797(83)90020-0. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Manning G. S. Thermodynamic stability theory for DNA doughnut shapes induced by charge neutralization. Biopolymers. 1980 Jan;19(1):37–59. doi: 10.1002/bip.1980.360190104. [DOI] [PubMed] [Google Scholar]
- Marx K. A., Ruben G. C. Evidence for hydrated spermidine-calf thymus DNA toruses organized by circumferential DNA wrapping. Nucleic Acids Res. 1983 Mar 25;11(6):1839–1854. doi: 10.1093/nar/11.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx K. A., Ruben G. C. Studies of DNA organization in hydrated spermidine-condensed DNA toruses and spermidine-DNA fibres. J Biomol Struct Dyn. 1984 Mar;1(5):1109–1132. doi: 10.1080/07391102.1984.10507507. [DOI] [PubMed] [Google Scholar]
- Nishio A., Uyeki E. M. Cellular uptake and inhibition of DNA synthesis by dihydroxyanthraquinone and two analogues. Cancer Res. 1983 May;43(5):1951–1956. [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Mazur S. J., Melançon P., Roe J. H., Shaner S. L., Unger L. Double helical DNA: conformations, physical properties, and interactions with ligands. Annu Rev Biochem. 1981;50:997–1024. doi: 10.1146/annurev.bi.50.070181.005025. [DOI] [PubMed] [Google Scholar]
- Traganos F. Dihydroxyanthraquinone and related bis(substituted) aminoanthraquinones: a novel class of antitumor agents. Pharmacol Ther. 1983;22(2):199–214. doi: 10.1016/0163-7258(83)90004-9. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Inhibition of cation-induced DNA condensation by intercalating dyes. Biopolymers. 1983 Jun;22(6):1621–1632. doi: 10.1002/bip.360220613. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Monomolecular condensation of lambda-DNA induced by cobalt hexamine. Biopolymers. 1983 Jun;22(6):1595–1620. doi: 10.1002/bip.360220612. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Schwarz G. Complex formation of acridine orange with single-stranded polyriboadenylic acid and 5'-AMP: cooperative binding and intercalation between bases. Biophys Struct Mech. 1979 Mar 21;5(1):75–90. doi: 10.1007/BF00535774. [DOI] [PubMed] [Google Scholar]


