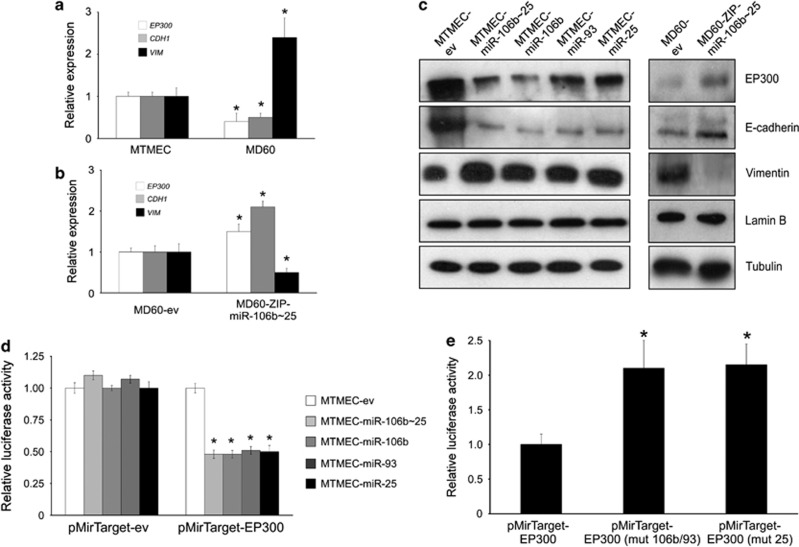
Figure 6.
The mir-106b∼25 cluster targets the E-cadherin transcriptional activator EP300. (a–c) Expression of the miR-106b∼25 cluster, or its individual miRs, leads to down-regulation of EP300, a transcriptional activator of E-cadherin expression. (a) Expression analysis by reverse transcription and real-time PCR normalized to the expression of RPS14 mRNA in doxorubicin-resistant cells. In drug-resistant MD60 cells, which over-express mir-106b∼25 cluster, both EP300 and CDH1 mRNAs are down-regulated, whereas VIM mRNA is up-regulated. (b) Rescue phenotype in cells stably transfected with a lentiviral ZIP construct to down-regulate endogenous miR-106b∼25 cluster expression. (c) Western blot showing down-regulation of EP300 and E-cadherin and up-regulation of vimentin in cells over-expressing the miR-106b∼25 cluster, or its individual miRs. Both lamin B and tubulin were used as loading controls. (d) The miR-106b∼25 cluster targets EP300. Normalized firefly luciferase activity from the reporter with the EP300 3′-UTR (pMirTarget-EP300) or the empty vector (pMirTarget-ev) after transient transfection. In all cases cells were co-transfected with a Renilla luciferase expression vector to normalize for transfection efficiency. Expression of each construct was normalized to that of MTMEC cells. (e) The putative miR target sequence in EP300 3′-UTR was mutated to abrogate miR-106b/93 (pMirTarget-EP300 (mut 106b/93)) or miR-25 binding (pMirTarget-EP300 (mut 25)) and used to transiently transfect MTMEC-miR-106b∼25 cells as in G. Numerical data show the average ±SD of at least three experiments (*P<0.05) and the immunoblot shows a representative of at least three replicates