Abstract
INTRODUCTION
Cavernous hemangiomas of the adrenal gland are rare. We report a case of a cavernous hemangioma of the adrenal gland presenting as an adrenal incidentaloma suspicious for adrenal cortical carcinoma (ACC).
PRESENTATION OF CASE
A 78 year old woman was admitted after a fall. Abdominal computed tomography revealed a large right adrenal lesion with features suspicious for adrenal cortical carcinoma (5.4 cm × 3.3 cm, unilateral, tumor calcifications, average Hounsfield units 55). The tumor was removed intact by a laparoscopic approach and pathology revealed a cavernous hemangioma of the adrenal gland.
DISCUSSION
Adrenal incidentalomas are found in up to 10% of patients undergoing abdominal imaging. Differential diagnosis includes both benign and malignant lesions. Guidelines for removal of adrenal incidentalomas recommend surgery based on functional status, size, and presence of concerning features on diagnostic imaging. Cavernous hemangiomas are rare, benign vascular malformations which can be challenging to distinguish pre-operatively from malignant lesions such as ACC.
CONCLUSION
Cavernous hemangiomas of the adrenal gland are exceedingly rare. These benign tumors have imaging features which may be suggestive of adrenal cortical carcinoma. The treatment of choice is surgical excision due the difficulty of excluding malignancy.
Keywords: Adrenal, Cavernous hemangioma, Adrenal cortical carcinoma
1. Introduction
Cavernous hemangiomas of the adrenal gland are rare benign tumors. Pathologically they are characterized by dilated vascular spaces with an endothelial lining showing areas of degeneration.1 We report a case of a cavernous hemangioma of the adrenal gland. It presented as an adrenal incidentaloma suspicious for adrenal cortical carcinoma (ACC). We review the literature on cavernous hemangiomas of the adrenal gland, focusing on the clinical presentation, imaging features and pathology of these lesions. We also highlight the challenges in distinguishing cavernous hemangiomas from ACC.
2. Presentation of case
A 78 female was admitted to hospital after falling down a flight of stairs. On presentation she had a slightly decreased Glasgow Coma Scale at 14, left sided facial droop and a blood pressure of 220/110 mmHg. She was taking no medications and a computed tomography (CT) scan of her head was normal. An X-ray of her right wrist showed an undisplaced distal radial fracture and a CXR revealed multiple rib fractures.
She was admitted to hospital and received treatment for her orthopedic injuries. During the admission she was found to have hyponatremia which resolved with treatment and recurrent falls. Six days after her admission she complained of chest and abdominal pain. This led to investigations including a CT scan of her chest and abdomen. In addition to her known rib fractures, she was found to have a large right adrenal lesion (5.4 cm × 3.3 cm) with areas of dystrophic calcification. This mass was well circumscribed with no apparent areas of invasion. There was no evidence of active bleeding or thrombus. The left adrenal was normal in appearance (Fig. 1a and b).
Fig. 1.
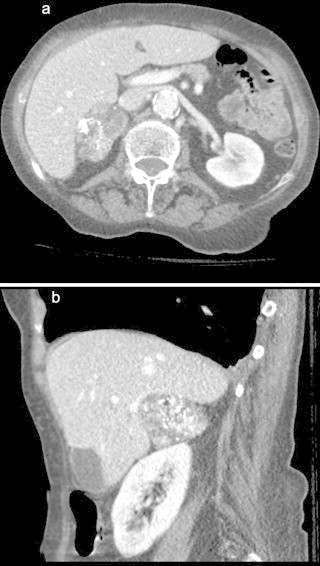
(a) Large right adrenal lesion (5.4 cm × 3.3 cm) containing regions of dystrophic calcification but well circumscribed with no obvious areas of invasion (a) axial and (b) sagittal. (b) Large right adrenal lesion (5.4 cm × 3.3 cm) containing regions of dystrophic calcification but well circumscribed with no obvious areas of invasion (a) axial and (b) sagittal.
The patient exhibited non-specific signs and symptoms including hypertension, weakness and osteopenia. She did not have clinical features overtly suggestive of a functioning adrenal tumor.
Functionality of the tumor was explored with a series of laboratory investigations (Table 1). An aldosterone secreting tumor was ruled out due to normal potassium and lack of hypertension. The diagnosis of pheochromocytoma was excluded due to normal levels of urinary catecholamines on 24 h collection. The low dose dexamethasone suppression test resulted in a mildly elevated cortisol, raising the possibility of sub-clinical Cushing's syndrome. Imaging characteristics were suspicious for ACC as the tumor was large (5.4 cm × 3.3 cm), unilateral, contained dystrophic calcifications, and had high density (HU 55). There was no overt evidence of invasion. The patient was then offered resection due diagnostic uncertainty and the possibility of malignancy. The tumor was approached laparoscopically via the transperitoneal approach, with a plan to convert to conventional, open surgery if any evidence of invasion was found. The decision with regard to approach to surgery is addressed in the discussion. The tumor was removed intact (Fig. 2), measuring almost 6 cm and was heterogeneous with multiple intratumoral cavities. The pathology showed a benign, well-encapsulated adrenal cavernous hemangioma (Fig. 3).
Table 1.
| Test | Patient value | Normal range |
|---|---|---|
| 24 h urinary normetanephrine | 1.4 μmol/d | 0.8–3.1 μmol/d |
| 24 h urinary metanephrine | 0.3 μmol/d | 0.2–0.9 μmol/d |
| 24 h urinary epinephrine | 59 pmol/L | 55–601 pmol/L |
| 24 h Urinary cortisol | 102.9 nmol/d | 25–220 nmol/d |
| Early morning serum cortisol (03:00) | 650 nmol/L | 275–555 nmol/L |
| Cortisol 1 mg overnight dexamethasone suppression test | 90 nmol/L | <80–140 nmol/L |
| Potassium | 4.2 mmol/L | 3.3–5.1 mmol/L |
Fig. 2.
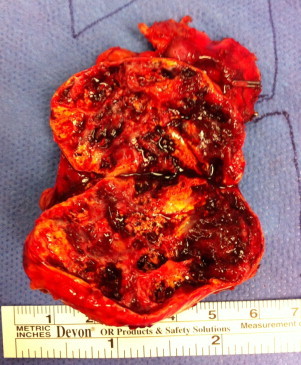
Resected adrenal gland showing a smooth surface and on sectioning an ill defined markedly congested lesion within which were isolated cystic spaces.
Fig. 3.
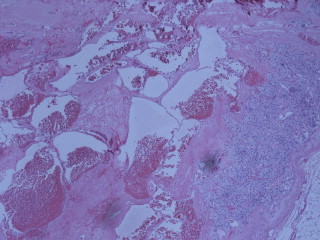
Thick walled, large interconnecting vascular channels filled with blood are present adjacent to the adrenal cortex. H&E 100×.
In follow up at three months after her operation, the patient is doing very well. Her incisions are healed with no evidence of herniation. No biochemical or radiographic follow up was conducted due to the benign pathology.
3. Discussion
Adrenal incidentalomas are adrenal tumors, greater than 1 cm, identified on imaging performed for other indications.2 Such lesions are found in up to 10% of patients undergoing abdominal imaging, leading to the growing problem of how these lesions should be further investigated and managed.2,3 Guidelines have been developed to aid clinicians and patients in determining which incidental adrenal masses should be excised. These guidelines incorporate lesion size, functional status and imaging features. Resection is generally advocated for all functioning lesions, as well as those with features suggestive of malignancy. These features include size over 4 cm (with thresholds ranging from 2.5 to 6 cm), density >20 HU, heterogeneity, delayed washout on enhanced CT images, or growth during observation.2
While 80% of adrenal incidentalomas are adrenal adenomas (70% of which are non-functional), the differential diagnosis of the incidental adrenal mass is broad. Other possible pathologies include malignant lesions such as adrenal cortical carcinoma (5%) and metastatic cancer from an extra-adrenal primary (2%), pheochromocytoma (5%), and benign lesions including adrenal cyst, myelolipoma, hematoma, ganglioneuroma or cavernous hemangioma.2
Cavernous hemangiomas of the adrenal are rare. Since the first cavernous hemangioma of the adrenal gland was surgically removed in 1955, only 58 further cases have appeared in the medical literature, to our knowledge.4 Of these, 55 were nonfunctional with two showing mineralcorticoid excess and one demonstrating glucocorticoid excess.4 Cavernous hemangiomas of the adrenal gland tend to be identified incidentally.1,5 There have also been rare cases of consumptive coagulopathy or hypovolemic shock due to hemorrhage leading to the diagnosis.6,7
While ultrasonography is generally not helpful in differentiating cavernous hemangiomas from other adrenal lesions, other imaging modalities are felt to be contributory.1,7 Adrenal hemangiomas tend to be heterogeneous, hypodense lesions with a high-density rim of tissue at the periphery of the lesion on enhanced CT.1,7 Characteristic calcifications have also been reported in 28–87% of cases.1 These represent phleboliths within the dilated vascular spaces of the lesion, and have been described as either speckled throughout the lesion or centrally located with an irregular, stellate branching pattern.1,5 Cavernous hemangiomas may also have peripheral crescentic calcifications.8 Although the irregular stellate pattern of calcifications is often discussed in a manner that hints at it being pathognomonic of adrenal cavernous hemangioma, calcifications lack specificity as are also seen in a variety of other adrenal lesions including ACC, hemorrhage, tuberculosis, neuroblastoma and have been reported in metastatic melanoma.1,8,9 Also, like ACC, cavernous hemangiomas tend to exhibit delayed washout of contrast on enhanced CT scans.5 The appearance on magnetic resonance imaging is that of a hypointense, heterogeneous lesion with a slightly hyperintense center on T1 images, and a high intensity peripheral rim on T2 images.6,7
On pathologic analysis, adrenal cavernous hemangiomas involve the adrenal cortex and consist of multiple dilated vascular channels lined by a single layer of vascular endothelium surrounded by a collagenous wall.5,7 Like their more common counterparts in the liver and skin, they generally contain areas of hemorrhage, necrosis, degeneration and calcification.5 The size of these lesions is variable, with many reported over 10 cm in size.6 The weight range of surgically excised specimens in the literature is from 140 to 5000 g.5 They tend to be unilateral, with only two cases of bilateral cavernous hemangiomas of the adrenal gland found on our search of the literature.5 While the etiology of these lesions is not known, they are thought to arise from the endothelial cells lining blood vessels. They are felt to be congenital with a natural history of enlargement over time due to vascular ectasia.1,7 Interestingly, there is some speculation that adrenal cavernous hemangioma are much more common than reported, but that they are misdiagnosed as (1) necrotic, nonfunctioning ACC on pathologic analysis due to inadequate assessment of the subcapsular area or (2) adrenal cysts due to loss of architecture from extensive necrosis and hemorrhage6,10. There has also been one reported case of coexisting malignant hemangioendothelioma.7
While laparoscopic adrenalectomy is considered a safe and effective approach for removal of benign lesions, controversy exists regarding minimally invasive resection in cases of suspected malignancy.2 The appropriateness of a laparoscopic approach in cases of possible malignancy continues to be a subject of debate in the surgical community.11 While some retrospective studies have shown no difference in recurrence rate, time to recurrence, or overall survival between laparoscopic and open approaches to ACC,12–14 others have found laparoscopic adrenalectomy to be associated with higher rates of peritoneal carcinomatosis, increased recurrence rates, incompleteness of resection and lowered overall survival.15,16 In this case, the laparoscopic approach was undertaken after significant consideration. The laparoscopic approach was felt to be reasonable due to (1) the lack of invasion on pre-operative imaging, and (2) the current state of equipoise in the literature with regard to laparoscopic versus open surgery in cases of suspected, but unproven, malignancy. Conversion to a conventional open surgery via an anterior approach was planned should invasion or confined malignancy be suspected during surgery. A posterior approach to resection was not considered because of concern that the patient may require open adrenalectomy with en bloc resection of involved structures should invasion be found. While the size of this lesion was only 5 cm, giant (>10 cm) cavernous hemangiomas of the adrenal gland have also been successfully resected using a laparoscopic approach, so size alone was not felt to be a factor in deciding between a laparoscopic or open surgery.7
4. Conclusion
Cavernous hemangiomas of the adrenal gland are rare. While there are certain features suggestive of the diagnosis, they fall short of being diagnostic. This lack of specificity in pre-operative studies often prevents a conclusive exclusion of malignancy from the differential diagnosis.17 The reported coexistence of cavernous adrenal hemangioma with malignant adrenal hemangioendothelioma further urges surgical resection even when the diagnosis of cavernous hemangioma is made. While in this case it was felt that a laparoscopic approach in experienced hands was a safe and effective option, with immediate conversion to an open approach should invasion become evident, we suggest individualizing the decision based on patient factors, tumor factors, and the experience of the operator. Caution should always be taken when embarking on a laparoscopic approach in the face of possible ACC, with a low threshold for conversion.
Conflicts of interest
None of the authors have any conflicts of interest to disclose.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
The manuscript was written by all the authors. Dr. Janet Edwards was involved in writing the paper and preparing the literature review. Dr. Heather Stuart was involved in complying patient history and diagnostic work-up. Processing and histologic analyses were done by Dr. Stefan Urbanski. Dr. Janice Pasieka is the attending surgeon.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Boraschi P., Campatelli A., Di Vito A., Perri G. Hemorrhage in cavernous hemangioma of the adrenal gland: US, CT and MRI appearances with pathologic correlation. European Journal of Radiology. 1995;21:41–43. doi: 10.1016/0720-048x(96)81068-0. [DOI] [PubMed] [Google Scholar]
- 2.Terzolo M., Stigliano A., Chiodini I., Loli P., Furlani L., Arnaldi G. AME position statement on adrenal incidentaloma. European Journal of Endocrinology/European Federation of Endocrine Societies. 2011;164:851–870. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 3.Paton B.L., Novitsky Y.W., Zerey M., Harrell A.G., Norton H.J., Asbun H. Outcomes of adrenal cortical carcinoma in the United States. Surgery. 2006;140:914–920. doi: 10.1016/j.surg.2006.07.035. discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 4.Oishi M., Ueda S., Honjo S., Koshiyama H., Yuba Y., Takabayashi A. Adrenal cavernous hemangioma with subclinical Cushing's syndrome: report of a case. Surgery Today. 2012;42:973–977. doi: 10.1007/s00595-012-0203-z. [DOI] [PubMed] [Google Scholar]
- 5.Auh Y.H., Anand J., Zirinsky K., Kazam E. Adrenal hemangioma: a case report. The Journal of Computed Tomography. 1986;10:57–59. doi: 10.1016/0149-936x(86)90012-3. [DOI] [PubMed] [Google Scholar]
- 6.Hamrick-Turner J.E., Cranston P.E., Shipkey F.H. Cavernous hemangioma of the adrenal gland: MR findings. Magnetic Resonance Imaging. 1994;12:1263–1267. doi: 10.1016/0730-725x(94)90091-5. [DOI] [PubMed] [Google Scholar]
- 7.Telem D.A., Nguyen S.Q., Chin E.H., Weber K., Divino C.M. Laparoscopic resection of giant adrenal cavernous hemangioma. JSLS: Journal of the Society of Laparoendoscopic Surgeons/Society of Laparoendoscopic Surgeons. 2009;13:260–262. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W.J., Weinreb J., Kumari S., Phillips G., Pochaczevsky R., Pillari G. Case report. Adrenal hemangioma. Journal of Computer Assisted Tomography. 1982;6:392–394. doi: 10.1097/00004728-198204000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Vargas A.D. Adrenal hemangioma. Urology. 1980;16:389–390. doi: 10.1016/0090-4295(80)90145-4. [DOI] [PubMed] [Google Scholar]
- 10.Ruebel A.A. Adrenal hemangioma. Urology. 1973;2:289–291. doi: 10.1016/0090-4295(73)90467-6. [DOI] [PubMed] [Google Scholar]
- 11.Creamer J., Matthews B.D. Laparoscopic adrenalectomy for cancer. Surgical Oncology Clinics of North America. 2013;22:111–124. doi: 10.1016/j.soc.2012.08.006. vi–vii. [DOI] [PubMed] [Google Scholar]
- 12.Lombardi C.P., Raffaelli M., De Crea C., Boniardi M., De Toma G., Marzano L.A. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery. 2012;152:1158–1164. doi: 10.1016/j.surg.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Porpiglia F., Fiori C., Daffara F., Zaggia B., Bollito E., Volante M. Retrospective evaluation of the outcome of open versus laparoscopic adrenalectomy for stage I and II adrenocortical cancer. European Urology. 2010;57:873–878. doi: 10.1016/j.eururo.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Brix D., Allolio B., Fenske W., Agha A., Dralle H., Jurowich C. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. European Urology. 2010;58:609–615. doi: 10.1016/j.eururo.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Leboulleux S., Deandreis D., Al Ghuzlan A., Aupérin A., Goéré D., Dromain C. Adrenocortical carcinoma: is the surgical approach a risk factor of peritoneal carcinomatosis? European Journal of Endocrinology/European Federation of Endocrine Societies. 2010;162:1147–1153. doi: 10.1530/EJE-09-1096. [DOI] [PubMed] [Google Scholar]
- 16.Miller B.S., Gauger P.G., Hammer G.D., Doherty G.M. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152:1150–1157. doi: 10.1016/j.surg.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Sabanegh E., Jr., Harris M.J., Grider D. Cavernous adrenal hemangioma. Urology. 1993;42:327–330. doi: 10.1016/0090-4295(93)90626-l. [DOI] [PubMed] [Google Scholar]


