Abstract
The degree of adherence to oral anti-cancer agents may influence treatment efficacy. Tamoxifen and toremifene usage data from two International Breast Cancer Study Group (IBCSG) studies were used to evaluate the impact of treatment adherence on the efficacy of these two selective estrogen receptor modulators (SERMs). Between 1993 and 1999 IBCSG Trial 13-93 randomized premenopausal women with node-positive disease to tamoxifen or no endocrine therapy and IBCSG Trial 14-93 compared tamoxifen and toremifene in postmenopausal women with node-positive disease. 690 women with estrogen-receptor positive (ER+) enrolled in these two trials were alive and disease-free 4 years after the start of SERM. The median follow up for this analysis was 9.0 years. Using a Kaplan-Meier landmark analysis at 4 years, the 609 women completing at least 4 years of SERM had improved 10-year disease-free survival (DFS) (71%) compared with the 81 women taking less than 4 years (64%), but these differences did not reach statistical significance [DFS hazard ratio (<4yr/4+yr) = 1.31, 95% CI (0.86, 1.98), p-value = 0.20]. Women who completed less than 4 years of SERM treatment (12%) appeared not to have achieved the full benefit from their therapy. These results suggest that more effort should be made to educate women regarding the benefits of a full course of treatment. This is especially important in light of the results of the recently reported ATLAS and aTTom trials which suggest a benefit of 10 years of tamoxifen.
Keywords: breast cancer, treatment adherence; tamoxifen; toremifene; selective estrogen receptor modulators; endocrine treatment
Introduction
Adherence to long-term oral cancer therapy can have a major impact on treatment efficacy (1-3), especially if unrecognised by healthcare providers. Adherence is defined as “the degree or extent of conformity to the recommendations about day-to-day treatment with respect to the timing, dosage, and frequency” (4) and is routinely used as synonymous with compliance, which can be associated too closely with blame. Another synonym for adherence is medication persistence, i.e. treatment continuation for the prescribed duration.
There currently is no consensus and limited data are available on the threshold for “adequate adherence” (5), with 80% being considered an acceptable cut-off (2). Adherence to long-lasting therapy for chronic illnesses in developed countries is on average 50%, but these reports do not usually include anticancer treatments (6, 7). Patient adherence to oral anticancer treatment is variable and difficult to predict (8, 9).
Patient-related, therapy-related, or healthcare system–associated factors may affect adherence. Age has been identified as one of the patient-related key factors linked with lower adherence (2, 10): older age (>75 years) (11-15) and younger age (<45 years) (2, 10, 16-18) are both significantly associated with poor adherence. In particular with regard to endocrine therapy for early breast cancer, most data for the general population are based on either self-reporting by patients, with the relative potential biases, or insurance/pharmacy databases recording medication refills (9): overall, adherence to adjuvant tamoxifen ranges from 25% to 96% (2, 10, 14, 19). Non-compliance to aromatase inhibitors (AIs) has been documented in patients treated outside clinical trials (16, 17, 20, 21). In the largest series report, during the first year, between 12 to 23% of women were non-adherent (22, 23), with mean adherence significantly decreasing with time (22).
The risk of reduced efficacy as a consequence of non-adherence is difficult to address. The relatively long half-life of tamoxifen (7-day to 14-day) (23) may not translate into reduced efficacy from occasionally missing tablets, while AIs have a much shorter half-life (approximately 2 days for letrozole and anastrozole and only 27 hours for exemestane) (24) with a greater potential detrimental effect on outcome from sporadic non-adherence.
Adherence of less than 80% (determined by prescription records) was associated with poorer survival at 2.4 years (HR 1.10, 95% CI =.001–1.21), measured as all-cause mortality, in a series of 1633 Irish women under tamoxifen (4). This observation was recently confirmed in a second series of 8,769 women from northern California, using either tamoxifen or AIs (25): adjusting for clinical and demographic variables, at median follow up of 4.4 years, both early discontinuation (HR 1.26, 95% CI 1.09-1.46) and non-adherence (HR 1.49, 95% CI 1.23-1.81) (measured by automated pharmacy records and prescription refill rates) among those who continued, were independent predictors of all-cause mortality.
When patients participating in clinical trials are non-adherent, inaccurate and flawed conclusions may result, possibly affecting therapeutic recommendations for the general population. Few data from randomized controlled trials are available (26). The NCIC MA.17 trial (extended adjuvant letrozole after 5 years of tamoxifen) reported reasons for discontinuation: patient refusal (11.4% letrozole; 11.1% placebo), toxicity (4.9% letrozole; 3.6% placebo), and “other reasons” (3.8% letrozole; 4.7%, placebo) (27). The NSABP B-14 trial (tamoxifen for up to 10 years) interestingly showed, in the first 5 years, therapy discontinuation was more frequent with placebo (13.0%) than tamoxifen (10.2%), suggesting that medication-related adverse events are not necessarily a reason for discontinuation (28). A meta-analysis of clinical studies (2) evaluating persistence with tamoxifen or an AI, reported that overall, 23% to 28% of patients followed for at least 4 years discontinued their oral endocrine therapy earlier than recommended. In the ATLAS trial, 2 years after entry, 84% of patients allocated to continue were still on tamoxifen compared with 4% of controls, a difference of 80% (31).
Patients and methods
Between 1993 and 1999 IBCSG conducted two complementary randomized adjuvant trials for pre- and postmenopausal patients with node-positive breast cancer evaluating the efficacy of a chemotherapy treatment gap and also addressing an endocrine therapy question (IBCSG trials 13-93 and 14-93). Trial 13-93 enrolled 1246 premenopausal women and studied the role of tamoxifen. Trial 14-93 accrued 969 postmenopausal patients and compared the efficacy of two SERMs, tamoxifen and toremifene. Following their cytotoxic treatment, 364 women in Trial 13-93 were randomized to receive tamoxifen and 393 women in Trial 14-93 were randomized to receive toremifene (N= 199) or tamoxifen (N=194). In addition, 199 women in Trial 14-93 were directly assigned to receive tamoxifen, due to the unavailability of toremifene in some countries. At median follow up of 7 years, the results of Trial 13-93 indicated that tamoxifen after adjuvant chemotherapy versus no tamoxifen significantly improved DFS in premenopausal patients with ER+ disease (HR =0.59; 95% CI, 0.46 to 0.75; P < .0001) (29).
The present short communication evaluates, in 956 women with ER+ disease randomized in IBCSG trials 13-93 and 14-93, the impact of receiving at least 4 years duration of SERM treatment. At each 6-month follow-up visit, SERM start and stop intervals, as well as start and stop dates for interruptions of more than one month were recorded by the clinician as reported by the patient. In this report “SERM adherence” was defined as the total number of months the patient reported receiving her assigned SERM. A cutoff of 4 years (48 months) was selected because very few patients discontinued their SERM prior to 4 years except due to recurrence. Preliminary analyses (not shown) found no difference in adherence according to the chemotherapy randomization, so results are presented separately by trial and SERM received.
Results
SERM treatment adherence is provided in Table 1, giving reasons that endocrine treatment was not begun or was stopped prior to completion, according to SERM group. Forty-two women (4%) did not begin their endocrine treatment. Nine-hundred-fourteen women (96%) began assigned SERM therapy and 305 (32%) discontinued prior to 48 months of treatment, of whom 106 (35%) stopped for reason other than recurrence or death unrelated to breast cancer. There was no difference among reasons for stopping early reported for tamoxifen and toremifene. The specific toxicities associated with stopping were not specified in the case report forms. The two groups, those completing 4 or more years of SERM and those stopping early did not differ with regard to the prognostic features of grade, age at diagnosis, tumor size and number of positive nodes. They also had similar first sites of recurrence, specifically local/regional versus distant metastatic sites.
Table 1.
Trials 13-93 and 14-93 SERM Treatment Adherence for Premenopausal (Trial 13-93) and Postmenopausal (Trial 14-93) Patients with ER-Positive Disease
| Tr 13-93 Randomized Tam | Tr 14-93 Assigned Tam | Tr 14-93 Randomized Tam | Tr 14-93 Randomized Tor | Total SERMs | |
|---|---|---|---|---|---|
| Total patients | 364 | 199 | 194 | 199 | 956 |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Patients recurring prior to starting SERM | 5 (1) | 1 (0.5) | 2 (1) | 2 (1) | 10 (1) |
| Patients not starting SERM | 16 (4) | 3 (2) | 3 (2) | 10 (5) | 32 (3) |
| Reasons for not starting assigned SERM | |||||
| Refusal | 12 (3) | 1 (0.5) | 1 (0.5) | - | 14 (1) |
| Medical decision | 1 (0.3) | - | 1 (0.1) | ||
| Received wrong arm | 2 (1) | 1 (0.5) | 1 (0.5) | 10 (5) | 14 (1) |
| Lost to follow up | - | - | 1 (0.5) | - | 1 (0.1) |
| Unknown | 1 (0.3) | 1 (0.5) | - | 2 (0.1) | |
| Patients starting SERM | 343 (94) | 195 (98) | 189 (97) | 187 (94) | 914 (96) |
| Patients with recurrence or unrelated death prior to completion of 4 yrs of SERM | 71 (20) | 36 (18) | 47 (24) | 45 (23) | 199 (21) |
| Patients discontinuing SERM prior to 4 yrs | 43 (12) | 17 (9) | 19 (10) | 27 (14) | 106 (11) |
| Reasons for discontinuation | |||||
| Toxicity | 21 (6) | 9 (5) | 13 (7) | 14 (7) | 57 (6) |
| Refusal | 8 (2 ) | 2 (1) | 4 (2) | 6 (3) | 20 (2) |
| Pregnancy desire | 5 (1) | - | - | 5 (1) | |
| Medical decision | - | 1 (1) | - | - | 1 (0.1) |
| Medical error | 2 (1) | - | - | 2 (0.2) | |
| Lost to follow up | 6 (2) | 4 (2) | 1 (1) | 6 (3) | 17 (2) |
| Unknown | 1 (0.3) | 1 (1) | 1 (1) | 1 (1) | 4 (0.4) |
| Landmark analysis cohort: Pts alive and disease free 4 years after SERM start | 263 (72) | 155 (78) | 139 (72) | 133 (67) | 690 (72) |
| Pts with 4+ yrs SERM | 229 (63) | 142 (71) | 123 (63) | 115 (58) | 609 (64) |
| Pts with < 4 yrs SERM | 34 (9) | 13 (7) | 16 (8) | 18 (9) | 81 (8) |
Percentages are of total N in each of the 4 treatment categories and total SERMs. Abbreviations: SERM: selective estrogen receptor modulator
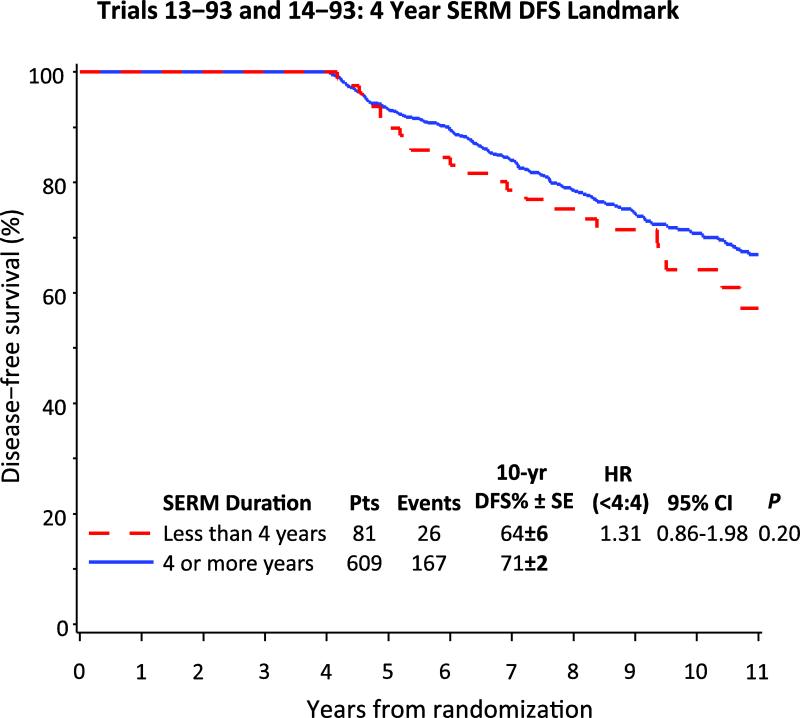
The median follow up for patients included in this evaluation was 9.0 years after enrollment. To avoid the “guarantee time” bias (i.e., in order to be adherent with treatment for 4 years a patient must be alive and disease-free for at least 4 years), a “landmark analysis” was performed on the cohort of 690 patients known to be alive and disease-free 4 years after study entry (30). DFS was defined as the length of time from the date of randomization to any relapse (including ipsilateral or contralateral breast recurrence), the appearance of a second primary malignancy, or death, whichever occurred first. Patients who received at least 4 years of SERM had better 10-year DFS (71% ± 2%) compared with the 81 patients who had less than 4 years (64% ± 6%) but the hazard ratio was not statistically significant [DFS HR (< 4 yr/ 4+ yr) = 1.31, 95% CI (0.86, 1.98), p-value = 0.20] (Figure 1).
Figure 1.
Disease-free survival from time of enrollment in IBCSG Trials 13-93 and 14-93, among 690 patients known alive and disease-free at 4 years, according to SERM adherence at 4 years.
Conclusions
Non-adherence to adjuvant endocrine therapy is increasingly recognized as an important factor influencing the disease outcome of women with early breast cancer. Insufficient adherence and follow-up data are available from most randomised clinical trials to document the prevalence and impact of adherence on DFS: in particular, rates are not always reported in the final published analysis, and reasons for discontinuation, when given, are often not clear. Published data show discontinuation rates generally not exceeding 25%. Based on data from almost 1000 women, our studies had a non-adherence rate of 15% which is comparable to previous observations (2, 10, 14, 19), with toxicity being the major reason for non-persistence (54%). A non-significant improved 10-year DFS was observed in patients taking at least 4 years of SERM, which is also consistent with available data in the general population (1, 23). Our data suggest non-adherence could influence reports of other trial results and subsequent recommendations for everyday clinical practice. As a consequence, consideration of adherence/persistence and routine prospective collection of treatment compliance should be incorporated in clinical trial design and conduct whenever new oral anticancer treatments are being evaluated. In recent years, extended endocrine therapy has been routinely considered for postmenopausal women with ER+ breast cancer, particularly among women with node-positive disease (27), the population evaluated in this report. In addition, the ATLAS (31) and aTTom (32) trials have recently reported a benefit of 10 years of tamoxifen: patients in our trials are however not comparable, being all node-positive, requiring chemotherapy and thus at higher risk of relapse. To obtain the maximum benefit of their long-lasting oral therapies, women should be more strongly encouraged and supported to complete their assigned treatment, although patients with small(er) risk of recurrence may gain little or only marginal benefit when considering treatment side effects and comorbidities. Strategies for patient education, i.e. increasing patients’ understanding of their disease and the appropriate strategies for good patient self-management, should be developed and implemented with the help of health care providers.
Acknowledgements
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group trials. This work was partially funded by the International Breast Cancer Study Group (IBCSG), which receives funding from the Swedish Cancer Society; The Cancer Council Australia; Australia and New Zealand Breast Cancer Trials Group (National Health and Medical Research Council); the Frontier Science and Technology Research Foundation; the Swiss Group for Clinical Cancer Research (SAKK); the Swiss Cancer League; and the United States National Institutes of Health (CA-75362).
Footnotes
Conflicts of Interest
None of the authors have conflicts of interest.
References
- 1.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. Br J Cancer. 2013;108:1515–1524. doi: 10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 4.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 7.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008 Apr;16(2) doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 9.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 10.Barron TI, Connolly R, Bennett K, et al. Early discontinuation of tamoxifen: A Lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 11.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 12.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: Predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 13.Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011;130:681–689. doi: 10.1007/s10549-011-1703-z. [DOI] [PubMed] [Google Scholar]
- 14.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 15.Kahn KL, Schneider EC, Malin JL, et al. Patient centered experiences in breast cancer: Predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431–439. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 16.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer. 2006;42:2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Fallowfield L, Atkins L, Catt S, et al. Patients' preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann Oncol. 2006;17:205–210. doi: 10.1093/annonc/mdj044. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Onc. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: A comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 20.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 21.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 22.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 23.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 24.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 25.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma S, Madarnas Y, Sehdev S, Martin G, Bajcar J. Patient adherence to aromatase inhibitor treatment in the adjuvant setting. Curr Oncol. 2011;18(Suppl 1):S3–9. doi: 10.3747/co.v18i0.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improved outcome in women with early stage breast cancer who completes 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. doi: 10.1200/JCO.2007.11.6798. [DOI] [PubMed] [Google Scholar]
- 28.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 29.International Breast Cancer Study Group. Colleoni M, Gelber S, Goldhirsch A, et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: IBCSG Trial 13-93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 30.Anderson JR, Cain KC, Gelber RD, et al. Analysis and interpretation of the comparison of survival by treatment outcome variables in cancer clinical trials. Cancer Treat Rep. 1985;69:1139–1146. [PubMed] [Google Scholar]
- 31.Davies C, Pan H, Goodwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 in 6,953 women with early breast cancer. J Clin Oncol. 2013;31(suppl) abstr 5. [Google Scholar]