Abstract
Introduction
We examined the efficacy of combination therapy with α1-blocker tamsulosin and hypnotic zolpidem in patients who had suffered from sleep disturbance associated with nocturia.
Material and methods
A total of 35 patients diagnosed with nocturia with lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) were studied. After treatment with tamsulosin for 4 weeks, 16 patients dissatisfied with nocturia (nocturiaquality of life index ≥4) and suspected to have sleep disturbance (Athens Insomnia Scale ≥6) received additional treatment with tamsulosin and zolpidem for 2 weeks. Outcomes were evaluated by the International Prostate Symptom Score (IPSS) and quality of life index (QOL), Athens Insomnia Scale (AIS) and nocturia-quality of life index (nocturia-QOL).
Results
After monotherapy with tamsulosin, significant reductions in IPSS (18.9 ±3.8 to 9.9 ±3.0, p <0.001), QOL (4.5 ±0.9 to 3.2 ±0.9, p <0.001) and nocturia episodes (3.4 ±0.7 to 2.6 ±1.0, p <0.001) were observed. However 20 patients were dissatisfied with nocturia (nocturia- QOL ≥4). Among 20 patients, 16 patients were suspected to have sleep disturbances (AIS ≥6). In these patients, additional therapy with tamsulosin and zolpidem significantly reduced nocturia episodes (3.3 ±0.8 to 1.9 ±0.7, p <0.001), AIS (10.6 ±2.9 to 6.8 ±25, p <0.001) and nocturia – QOL (5.6 ±0.5 to 3.6 ±1.1, p <0.001) compared with patients after treatment with tamsulosin only.
Conclusions
Combination therapy with tamsulosin and zolpidem may be useful for patients with BPH dissatisfied with nocturia and suspected to have sleep disturbance.
Keywords: nocturia, tamsulosin, zolpidem, benign prostatic hyperplasia
INTRODUCTION
Nocturia is defined as waking at night to void urine, and nocturia has been reported in 58-90% of people over 50 years of age, with the prevalence of nocturia increasing with age. This condition can significantly impair a patient's perception of their well being and often warrants treatment, especially when the patient must wake twice or more per night [1–5].
Nocturia is associated with impaired health, deterioration in sleep, and quality of life. It has also been associated with an increased risk of death after adjusting for age, cardiac disease, diabetes mellitus and stroke [6]. The increased mortality may to some extent be explained by an increased risk of falls, which is one consequence of nocturia [7, 8]. A quarter of the falls occur at night, and more than a quarter of these nocturnal falls occur during trips to the toilet [9]. Nocturia is often attributed to benign prostatic hyperplasia (BPH) in men, but a causal relation is uncertain, although the two conditions may be associated in older individuals. Nocturia is often found to respond less satisfactorily than other lower urinary tract symptoms (LUTS) after medical or surgical treatment of BPH [10]. Primary sleep disturbance may be a result of arousals with voiding. Sleep disturbance is a common experience of older adults, many of whom depend on prescribed sedatives [11]. Although one report outlines the improvement of nocturia through the use of a hypnotic agent, there are no reports, to our knowledge, that address the actual effect of hypnotic agents on patients with nocturia after a diagnosis of insomnia [14].
The aim of the present study was to determine whether zolpidem, a nonbenzodiazepine hypnotic agent, would be useful for patients dissatisfied with nocturia and suspected to have sleep disturbance after monotherapy with tamsulosin.
METHODS
A total of 35 male outpatients (age ≥50 years) with a complaint of nocturia with LUTS suggestive of BPH were enrolled in the present study from September 2005 to August 2006. Informed consent was obtained from each patient. The enrolled patients experienced 2 or more episodes of nocturia per night and had a nocturia-quality of life (QOL) score of 4 or more. Nocturia – QOL score (Table 1) was used as a simple tool to measure the satisfaction of patients with symptoms of nocturia. Each patient's medical history was taken, and a physical examination was performed. Urinalysis and urine culture were performed to rule out urinary tract infection. Blood samples were taken for measurement of prostate-specific antigen (PSA). Uroflowmetry studies were performed on each patient according to the criteria of the International Continence Society. Abdominal ultrasonography was performed on each patient to determine prostate volume and post-voided residual and to rule out any urinary system abnormality. Patients with prostate cancer, abnormal prostate findings on digital rectal examination (nodularity or induration), or PSA values of ≥4 ng/mL were excluded from the study. The exclusion criteria also included the use of medications for the control of bladder symptoms and use of hypnotics.
Table 1.
Nocturia-QOL score nocturia
| Delighted | Pleased | Mostly Satisfied | Mixed: about equally satisfied and dissatisfied | Mostly Dissatisfied | Unhappy | Terrible | |
|---|---|---|---|---|---|---|---|
| If you were to spend the rest of your life with your symptoms of nocturia just the way they are now, how would you feel about that? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
QOL – quality of life
The patients were assessed using the score for the seventh question of the International Prostate Symptom Score (IPSS), the Athens Insomnia Scale (AIS) and the nocturia-QOL score. The AIS, an instrument that measures the intensity of sleep-related problems, was used as a screening tool for the diagnosis of insomnia. Sleep disturbance is suspected when the score is ≥6.
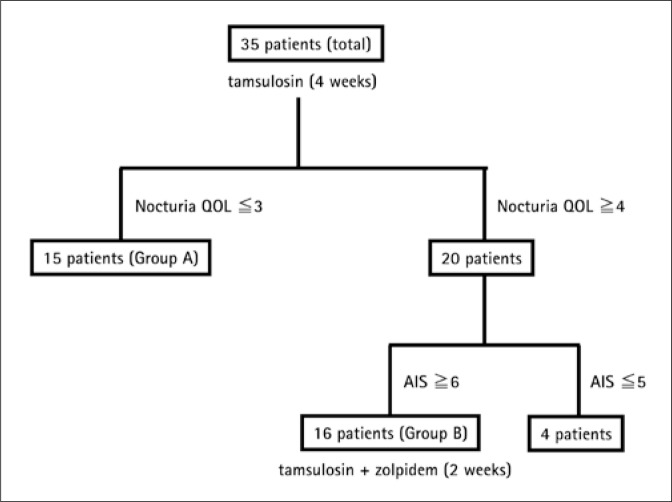
Enrollment and follow-up of patients in the present study are shown in Figure 1. After assessment at baseline, the 35 patients were administered tamsulosin (0.2 mg/day) for 4 weeks before re-assessment. A nocturia-QOL score of ≤3 was regarded as indicating patient satisfaction with nocturia (defined as Group A), and a nocturia-QOL score of ≥4 was regarded as indicating patient dissatisfaction with nocturia. Patients dissatisfied with nocturia and with sleep disturbances (AIS ≥6) received additional treatment with zolpidem 5-10 mg once per night for 2 weeks (defined as Group B).
Fig. 1.
Enrollment and follow-up of patients.
Statistical analyses were performed with JMP® version 5.01 statistical analysis software. Data were compared for statistical significance using the Wilcoxon signed-rank test. All values are expressed as the mean (SD), with statistical significance inferred at a value of p <0.05 unless otherwise specified.
RESULTS
Demographic and other baseline characteristics of all patients (n = 35) and Group A (n = 15) and Group B (n = 16) patients are presented in Table 2. The mean age of all patients was 70.3 ±9.6 years. Mean prostate volume was 19.5±9.8 ml, and mean max flow rate and post-voided residual were 11.3 ±3.6 ml/s and 47.2 ±29.8 ml, respectively. Differences in mean values of age, prostate volume, max flow rate and post-voided residual were not significant between Group A and Group B.
Table 2.
Demographics and other baseline characteristics
| All patients (n = 35) | Group A (n = 15) | Group B (n = 16) | p-value A vs. B | |
|---|---|---|---|---|
| Age (years) | 70.3±9.6 | 68.9±4.6 | 73.2±5.1 | NS |
| Prostate volume (ml) | 19.5±9.8 | 20.6±4.8 | 18.8±3.1 | NS |
| Max flow rate (ml/s) | 11.3±3.6 | 10.6±2.9 | 11.7±3.4 | NS |
| Post-voided residual (ml) | 47.2±29.8 | 56.3±32.3 | 49.8±31.2 | NS |
NS - not significant (Mann-Whitney U test)
The score at baseline and post-treatment for the IPSS, QOL index, nocturia episodes, nocturia-QOL and AIS are shown in Table 3. In all patients, monotherapy with tamsulosin for 4 weeks significantly improved IPSS (18.9 ±3.8 to 9.9 ±3.0, p <0.001), QOL (4.5 ±0.9 to 3.2 ±0.9, p <0.001), nocturia episodes (3.4 ±0.7 to 2.6 ±1.0, p <0.001), nocturia-QOL (5.5 ±0.6 to 4.3 ±1.5, p <0.001) and AIS (11.7 ±3.6 to 9.1 ±3.1, p ≤0.001).
Table 3.
Effects of tamsulosin/zolpidem on nocturia in patients with benign prostatic hyperplasia
| IPSS | QOL | Nocturia episodes | Nocturia QOL | AIS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 35) | Baseline | 18.9 ±3.8 | ** | 4.5 ±0.9 | ** | 3.4 ±0.7 | ** | 5.5 ±0.6 | ** | 11.7 ±3.6 | ** |
| After treatment | 9.9 ±3.0 | 3.2 ±0.9 | 2.6 ±1.0 | 4.3 ±1.5 | 9.1 ±3.1 | ||||||
| Group A (n = 15) | Baseline | 18.3 ±3.7 | ** | 4.4 ±0.6 | ** | 3.3 ±0.6 | ** | 5.2 ±0.6 | ** | 11.2 ±3.6 | ** |
| After treatment | 8.7 ±3.2 | 2.5 ±0.6 | 1.7 ±0.5 | 2.6 ±0.5 | 7.9 ±3.1 | ||||||
| Group B (n = 16) | Baseline | 18.9 ±4.1 | ** | 4.6 ±1.0 | ** | 3.4 ±0.9 | NS | 5.7 ±0.5 | NS | 12.9 ±3.3 | ** |
| After treatment | 10.4 ±2.6 | 3.8 ±0.6 | 3.3 ±0.8 | 5.6 ±0.5 | 10.6 ±2.9 | ||||||
| After additional treatment | 9.3 ±2.6 | NS | 3.5 ±1.4 | NS | 1.9 ±0.7 | ** | 3.6 ±1.1 | ** | 6.8 ±2.5 | ** | |
IPSS - International Prostate Symptom Score, QOL - quality of life, AIS - Athens Insomnia Scale, All and Group A - Monotherapy with tamsulosin for 4 weeks, Group B - Combination therapy with tamsulosin and zolpidem for 2 weeks, After treatment - after treatment with tamsulosin, After additional treatment - after treatment with tamsulosin and zolpidem.
p < 0.001, NS: p ≥0.05 (Wilcoxon signed-rank test)
Among all patients, a reduction in the nocturia-QOL score (≤3) was observed in 15 patients (Group A), but 20 patients were not satisfied with symptoms of nocturia (nocturia-QOL ≥4). In Group A, monotherapy with tamsulosin significantly reduced the IPSS (18.3 ±3.7 to 8.7 ±3.2, p <0.001), QOL (4.4 ±0.6 to 2.5 ±0.6, p <0.001), nocturia episodes (3.3 ±0.6 to 1.7 ±0.5, p <0.001) and AIS (11.2 ±3.6 to 7.9 ±3.1, p <0.001).
Among the 20 patients not satisfied with symptoms of nocturia (nocturia-QOL ≥4), 16 patients (Group B) were suspected of having sleep disturbances (AIS ≥6). In Group B, treatment with tamsulosin only did not result in a significant reduction in nocturia episodes (3.4 ±0.9 to 3.3 ±0.8) and nocturia-QOL score (5.7 ±0.5 to 5.6 ±0.5), although significant reductions in IPSS (18.9 ±4.1 to 10.4 ±2.6, p <0.001), QOL (4.6 ±1.0 to 3.8 ±0.6, p <0.001) and AIS (12.9 ±3.3 to 10.6 ±2.9, p <0.001) were observed. After additional treatment with tamsulosin and zolpidem for 2 weeks, significant decreases in nocturia episodes (3.3 ±0.8 to 1.9 ±0.7, p <0.001), nocturia-QOL (5.6 ±0.5 to 3.6 ±1.1, p <0.001) and AIS (10.6 ±2.9 to 6.8 ±2.5, p <0.001) were observed compared with those after treatment with tamsulosin only. No serious adverse effects were observed in patients treated with tamsulosin and zolpidem.
DISCUSSION
BPH can cause bladder outlet obstruction and induce secondary bladder overactivity and reduction of functional bladder capacity, which may result in filling symptoms including nocturia. Therefore, urologists often treat patients whose chief symptom is nocturia with modalities for BPH, including conservative medical treatment and surgical intervention [12]. In regard to degrees of improvement, nocturia is the lowest among the seven individual symptoms included in the IPSS. Unlike the other six symptoms, nocturia is markedly influenced by various factors other than BPH, and other approaches might be required for the treatment of nocturia [10, 12].
Nocturia is associated with various factors of pathologic conditions such as cardiovascular disease and diabetes mellitus, anxiety and primary sleep disorders, and behavioral and environmental factors. The underlying pathophysiologic process of nocturia consists of three main conditions: polyuria, nocturnal polyuria, and bladder storage problems. However, nocturia can be secondary to awakening and sleep disturbances rather than to nocturnal polyuria in patients with sleep disturbances from other causes [3].
The present study showed that monotherapy with tamsulosin significantly improved the IPSS and QOL scores, and a reduction in the nocturia-QOL score (≤3) was observed in 15 (43%) Group A patients. Interestingly, a significant improvement in AIS was also seen in Group A. Djavan et alreported that tamsulosin improves nocturia and sleep disturbances (by increasing the number of hours of undisturbed sleep) in LUTS/BPH [13]. Thus, the results of the present study suggest that the improvement of nocturia by monotherapy with tamsulosin may improve sleep disturbances. The AIS was developed as a brief and easily administered selfassessment questionnaire for estimating the severity of insomnia encountered in a large variety of clinical and research settings [17]. The AIS can be used in clinical practice and research, not only to reliably measure the intensity of sleep difficulty, but also to assist in establishing the diagnosis of insomnia. When diagnosing individuals with a score of 6 or higher as insomniacs, the scale has a sensitivity of 93% and specificity of 85% [18].
Subjectively, sleep disturbances were observed in 80% (16/20) of patients who did not show a decrease in nocturia-QOL score of ≤3) by monotherapy with tamsulosin. In these patients (Group B), additional 2-week combination therapy with tamsulosin and zolpidem significantly reduced nocturia episodes, AIS and nocturia-QOL score, indicating that improvement of sleep disturbances may contribute to improve symptoms of nocturia. In addition, these results suggest that improvement in the number of nocturia episodes may be closely related to improvement in the nocturia-QOL score. Recently, Song and Ku showed that zolpidem improved the frequency of nocturia unresponsive to treatment by an alpha-blocker (terazosin or tamsulosin) in men with LUTS [14].
In the present study, patients were selected in whom sleep disturbance appeared to be a cause of nocturia after evaluation using the nocturia-QOL score and the AIS. The nocturia-QOL score is a simple and useful tool for evaluating disease-specific QOL related to nocturia.
A problem identified in the present study was that objective efficacy, determined by assessing nocturia episodes from the frequency-volume chart, was not evaluated. There may be poor agreement between the subjectively estimated nocturnal frequency from the IPSS and chart-determined nocturnal frequency. However, the change in QOL related to nocturnal frequency could be evaluated with the nocturia-QOL score.
Yoshimura et al. reported that sleep and general-related QOL in the mildly nocturic patient were more greatly affected by trouble sleeping due to night-time frequency than by the frequency of nocturnal urinary voiding per se [12]. They suggest that the strategy for treatment of nocturia may be improved if urologists clearly distinguish whether nocturic patients are bothered by trouble sleeping. Therefore, we believe that it is beneficial to use the nocturia-QOL score and AIS to identify the cause of deterioration in QOL by nocturia-related sleep disturbance and to treat with hypnotic agents.
The present study is limited in that we could not directly compare the effect of combination therapy (tamsulosin with zolpidem) with monotherapy (tamsulosin alone) in Group B after monotherapy with tamsulosin for 4 weeks. However, after additional treatment with tamsulosin and zolpidem for 2 weeks, significant decreases in episodes of nocturia and nocturia-QOL and AIS scores were observed, whereas IPSS and QOL scores did not decrease significantly. Therefore, the effect is not due to the longer exposure to tamsulosin but rather to the addition of zolpidem.
In this study, patients were given 0.2 mg tamsulosin once daily. A 0.4-mg dose of tamsulosin is routinely used in North America and Europe to treat BPH. However, previous studies show that lower doses of tamsulosin than those used in Western countries are equally effective for treating symptomatic BPH in Asian patients [19].
Hypnotics such as zolpidem may be useful as additional treatment in patients unresponsive to treatment by an alpha 1-blocker such as tamsulosin in patients in whom the etiology of nocturia is associated with sleep disturbances. The zolpidem used in the present study has a low propensity to cause clinical next-day residual effects, withdrawal, dependence or tolerance [15]. In animal studies, zolpidem has weaker myorelaxant and anticonvulsant effects than the benzodiazepines because of the selectivity of zolpidem for benzodiazepine ω1 receptors [16]. Yokoyama et al. reported that zolpidem increased bladder capacity via a GABA-ergic mechanism in cerebral infarction rats and suppressed urine excretion via a pathway that was not through activation of vasopressin V2 receptors in water-loaded and Brattleboro rats [20]. Therefore, zolpidem may improve nocturia via an increase in bladder capacity and a decrease in urine excretion. In the present study, no remarkable side effects or voiding disturbances were reported. Therefore, we believe that zolpidem is considered safe for BPH patients and is suitable for use as a hypnotic agent in patients complaining of nocturia.
CONCLUSION
Nocturia is associated with various factors including sleep disturbance. The AIS is useful for evaluating patients with sleep disturbance that appears to be a cause of nocturia. The effectiveness of tamsulosin on nocturia in patients with BPH is limited when the etiology of nocturia is associated with sleep disturbances. In such patients, hypnotic agents such as zolpidem may be useful as an additional treatment.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Homma Y, Kakizaki H, Gotoh M, et al. Epidemiologic survey on lower urinary tract symptoms in Japan (in Japanese) J Neurogenic Bladder Soc. 2003;14:266–277. [Google Scholar]
- 3.van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 4.Asplund R, Aberg H. Health of the elderly with regard to sleep and nocturnal micturition. Scand J Prim Health Care. 1992;10:98–104. doi: 10.3109/02813439209014044. [DOI] [PubMed] [Google Scholar]
- 5.Jolleys JV, Donovan JL, Nanchahal K, Peters TJ, et al. Urinary symptoms in the community: how bothersome are they? Br J Urol. 1994;74:551–555. doi: 10.1111/j.1464-410x.1994.tb09182.x. [DOI] [PubMed] [Google Scholar]
- 6.Asplund R. Sleep and hypnotics among the elderly in relation to body weight and somatic disease. J Intern Med. 1995;238:65–70. doi: 10.1111/j.1365-2796.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 7.Stewart RB, Moore MT, May FE, et al. Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc. 1992;40:1217–1220. doi: 10.1111/j.1532-5415.1992.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 8.Tideiksaar R. Preventing falls: how to identify risk factors, reduce complications. Geriatrics. 1996;51:43–46. [PubMed] [Google Scholar]
- 9.Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Falls among frail older people in residential care. Scand J Public Health. 2002;30:54–61. [PubMed] [Google Scholar]
- 10.Homma Y, Yamaguchi T, Kondo Y, et al. Significance of nocturia in the International Prostate Symptom Score for benign prostatic hyperplasia. J Urol. 2002;167:172–176. [PubMed] [Google Scholar]
- 11.Drake MJ, Mills IW, Noble JG. Melatonin pharmacotherapy for nocturia in men with benign prostatic enlargement. J Urol. 2004;171:1199–1202. doi: 10.1097/01.ju.0000110442.47593.ea. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura K, Ohara H, Ichioka K, et al. Nocturia and benign prostatic hyperplasia. Urology. 2003;61:786–790. doi: 10.1016/s0090-4295(02)02444-5. [DOI] [PubMed] [Google Scholar]
- 13.Djavan B, Milani S, Davies J, Bolodeoku J. The Impact of Tamsulosin Oral Controlled Absorption System (OCAS) on Nocturia and the Quality of Sleep: Preliminary Results of a Pilot Study. Eur Urol Supplements. 2005;4:61–68. [Google Scholar]
- 14.Song YS, Ku JH. Zolpidem pharmacotherapy combined with alpha-blocker therapy for nocturia unresponsive to alpha-blocker monotherapy in men with lower urinary tract symptoms: a preliminary study. Int Urol Nephrol. 2007;39:1147–1152. doi: 10.1007/s11255-007-9206-x. [DOI] [PubMed] [Google Scholar]
- 15.Swainston Harrison T, Keating GM. Zolpidem: a review of its use in the management of insomnia. CNS Drugs. 2005;19:65–89. doi: 10.2165/00023210-200519010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Depoortere H, Zivkovic B, Lloyd KG, et al. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. J Pharmacol Exp Ther. 1986;237:649–658. [PubMed] [Google Scholar]
- 17.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 18.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55:263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 19.Okada H, Kamidono S, Yoshioka T, et al. A comparative study of terazosin and tamsulosin for symptomatic benign prostatic hyperplasia in Japanese patients. BJU Int. 2000;85:676–681. doi: 10.1046/j.1464-410x.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama O, Matsuta Y, Yanai-Inamura H, et al. Zolpidem increases bladder capacity and decreases urine excretion in rats. Neurourol Urodyn. 2010;29:587–591. doi: 10.1002/nau.20797. [DOI] [PubMed] [Google Scholar]