Abstract
In order to examine the toxicity profile of glycine, an authorized food additive, a solution of glycine in water for injection was administered orally (via gavage) to male SD rats (Crl:CD(SD)) once daily for 4 weeks at doses of 500, 1000 and 2000 mg/kg/day in a volume of 10 mL/kg. Control animals received vehicle only. No animals died, and no glycine-related changes were observed in body weight, food consumption, water consumption, hematology, organ weight, gross pathological examination or histopathological examination. In urinalysis, daily urinary volume and urinary Cl excretion were significantly higher in the 2000 mg/kg/day dose group, and urine pH and urinary protein showed lower trends in the glycine-treated groups. However, these changes were considered to be of little toxicological significance, because there were no histopathological changes in the kidneys or urinary bladder and no changes in other urinary parameters. As regards blood chemistry, phospholipids were significantly higher in the 2000 mg/kg/day dose group. However, the increase was small and was not considered to be toxicologically significant. In conclusion, none of the animals in any of the glycine-treated groups showed changes that were considered toxicologically significant. Therefore, the no-observed-adverse-effect level of glycine was estimated to be at least 2000 mg/kg/day under the conditions of this study.
Keywords: glycine, amino acid, toxicity, rat
Introduction
Glycine, the only amino acid having no asymmetric carbon, is the smallest of the 20 amino acids commonly found in proteins, and is not an essential amino acid, as it is biosynthesized in the body from serine. Pharmacologically, glycine acts as an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. Due to its flavor-enhancing, antimicrobial, chelating and buffering actions, glycine is widely used as a food additive in Japan. In recent years, glycine has also come to be used as a supplement, such as a sleep aid.
There have been a relatively small number of toxicity studies of glycine, possibly because glycine is thought to be a highly safe substance1. The oral LD50 in rats is high (7930 mg/kg)2 and results of genotoxicity studies are negative3. In contrast, necrotic and neoplastic changes in renal papillae were reported in a carcinogenicity study in rats4. However, its toxicity profile has not yet been comprehensively established, and no reports were found regarding oral (via gavage) repeated-dose general toxicity studies of glycine. Therefore, the aim of the present study was to further examine the toxicity of glycine by means of a 4-week, repeated-dose gavage administration study in rats.
Materials and Methods
Animals and animal husbandry
Five-week-old male Sprague-Dawley rats (Crl: CD (SD)) were purchased from Charles River Laboratories Japan Inc. (Kanagawa, Japan) and allowed ad libitum access to tap water and a gamma-ray sterilized powder diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan). All animals were housed individually in metallic bracket cages in a breeding room kept at temperature of 20 to 26°C and a relative humidity of 30 to 70%, with an air ventilation cycle of 10 to 15 times/hour (all-fresh air ventilation) and a 12-hour light/dark cycle (light on from 7 a.m. to 7 p.m.). They were allowed to acclimate for 6 days prior to experimentation and then were randomly allocated to four groups. Body weight at the start of experiments was in the range of 193.9 to 215.1 g. All animals were treated humanely according to institutional guidelines, and the experimental procedure was approved by the institutional ethics committee.
Test article and dosage preparation
Glycine (lot M5G2082; purity 100.0%) was purchased from Nacalai Tesque, Inc. (Kyoto, Japan), and stored in an airtight container at room temperature. Water for injection (D.W., lot 5A88; Otsuka Pharmaceutical Factory Inc., Tokushima, Japan) was used as the vehicle and negative control. Glycine solution in D.W. was prepared once a day at the time of use.
Treatment
Three groups of 6 male rats were treated with glycine solution once daily by oral gavage at doses of 500, 1000, or 2000 mg/kg/day in a volume of 10 mL/kg for 28 consecutive days. The high-dose level was set at 2000 mg/kg/day, which is the maximum dose concentration in oral gavage recommended in the guideline 5. The middle- and low-dose levels were set at 1000 and 500 mg/kg/day at a common ratio of 2. Animals in the control group were given the vehicle only. The duration of administration was set at four weeks in order to obtain information on the possible health hazards likely to arise from repeated exposure over a relatively limited period of time.
Clinical evaluations
All animals were examined twice daily (pre-dose and post-dose) for clinical signs. Individual body weights were recorded for all animals on days 1, 3, 8, 15, 22 and 28 and on the day of necropsy. Food consumption and water consumption on days 2–3, 7–8, 14–15, 21–22 and 27–28 was measured individually for all animals.
Clinical laboratory evaluations
Urinalysis, hematology and blood chemistry parameters were evaluated on the day of scheduled necropsy. Urine samples were collected for approximately 18 hours from individual animals placed in metabolism cages. Urine volume (U. Vol), specific gravity (S.G., digital urine gravity refractometer), pH, glucose, protein, occult blood, ketone, bilirubin and urobilinogen were measured using an automated urine analyzer (CliniTek 500, Bayer Medical), and total amounts of sodium, potassium and chloride ions excreted were measured using a fully automatic electrolyte analyzer (A&D, EA06T). Blood samples were collected from the caudal vena cava under ether anesthesia prior to necropsy for hematology and blood chemistry. Blood samples containing EDTA-2K as an anticoagulant were analyzed for red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count and white blood cell count (WBC) using a multi blood testing device (THMS H1E, Bayer Sankyo). Blood smears were prepared using an automatic centrifugal concentrator (806-0400, Hitachi) and stained with May-Grunwald Giemsa using an automatic stainer (806-0100, Hitachi) for differential white blood cell counts, including neutrophil count and percentage (NEUT), lymphocyte count (sum of the lymphocyte count and large unstained cell count) and percentage (LYM), monocyte count and percentage (MONO), eosinophil cell count and percentage (EOS) and basophil count and percentage (BASO). Reticulocytes were counted using an automated reticulocyte analyzer (R-3500, Sysmex). Blood samples anticoagulated with trisodium citrate were used for measurement of prothrombin time (PT), activated partial thromboplastin time (APTT) and fibrinogen (Fbg) with a fully automated blood coagulation analyzer (CA-5000, Sysmex). Blood plasma samples obtained from heparinized blood were evaluated for lactic dehydrogenase (LDH), creatine phosphokinase (CPK), glutamate and oxaloacetate transaminase (GPT), alkaline phosphatase (ALP), total bilirubin (T-BIL), creatinine (CRE), urea nitrogen (BUN), triglyceride (TG), total cholesterol (T-CHO), phospholipid (PL), glucose (GLU), sodium ion (Na), potassium ion (K), chloride ion (Cl), calcium (Ca), inorganic phosphorus (IP) and total protein (TP) using an automatic analyzer for clinical chemistry (TBA-120FR, Toshiba). Serum protein was examined with a Rapid ElectroPhoresis system (Helena Laboratories, Beaumont, TX, USA) to determine the albumin/globulin ratio (A/G), albumin (ALB), α1 globulin ratio (ALPHA1), α2 globulin ratio (ALPHA2), beta globulin ratio (BETA) and gamma globulin ratio (GAMMA).
Postmortem evaluations
On the day after the last treatment, all animals were euthanized by exsanguination from the abdominal aorta under ether anesthesia and were subjected to a complete gross pathological examination, including external appearance and the peritoneal, pleural and cranial cavities. Although the use of ether as an anesthetic in research animals is now strongly discouraged, the experiment in this report was performed in 2005, when ether was a more general anesthetic than today. The following organs were excised and weighed: brain, pituitary, thymus, submaxillary glands (Submax.G., submandibular glands and sublingual glands), heart, spleen, liver, kidneys, adrenal glands, and testes. The tissues and organ samples were the treated as follows. Neutral buffered 10% formalin solution was used for fixation and preservation except for the testes, which were processed in Bouin’s fixative. Tissues were prepared for histopathological examination by embedding in paraffin wax, sectioning and staining with hematoxylin and eosin. Tissues and organs examined were skin, mammary gland, cerebrum, cerebellum, adrenal glands, spinal cord, lungs, thymus, sublingual gland, submandibular gland, tongue, exorbital lacrimal gland, mandibular lymph node, liver, heart, spleen, kidneys, stomach (forestomach and glandular stomach), duodenum, pancreas, jejunum, ileum, cecum, colon, mesenteric lymph node, thyroid glands, parathyroid glands, trachea, esophagus, pituitary, aorta, skeletal muscle, sciatic nerve, eyes, Harderian glands, urinary bladder, prostate, seminal vesicles, coagulating gland, testes, epididymides, bone marrow (sternum) and auricle on the side of an ear tag.
Data analysis
Body weight, food consumption, water consumption, urinalysis, hematology, blood chemistry, and organ weight data were recorded using a MiTOX RDT system. Numerical data obtained during the study were used to calculate group mean values and standard deviations. Group variances for the appropriate parameters were compared using Bartlett’s method. When the differences between group variances were not significant, Dunnett’s multiple comparison method was applied to determine the significance of differences between the control group and each glycine-treated group. If the Bartlett’s test indicated significant differences between group variances for a given parameter, that parameter was compared among groups using the Steel’s multiple comparison method for mean ranking. The criterion of significance was an alpha level of 0.05 or 0.01.
Results
There were no deaths during the administration period in any of the study groups. No clinical signs were observed during the 4-week administration period. There were no significant differences in body weight (Table 1), food consumption (Table 2) and water consumption (Table 3) between the glycine-treated groups and the control group.
Table 1. Body Weight (Unit: g).
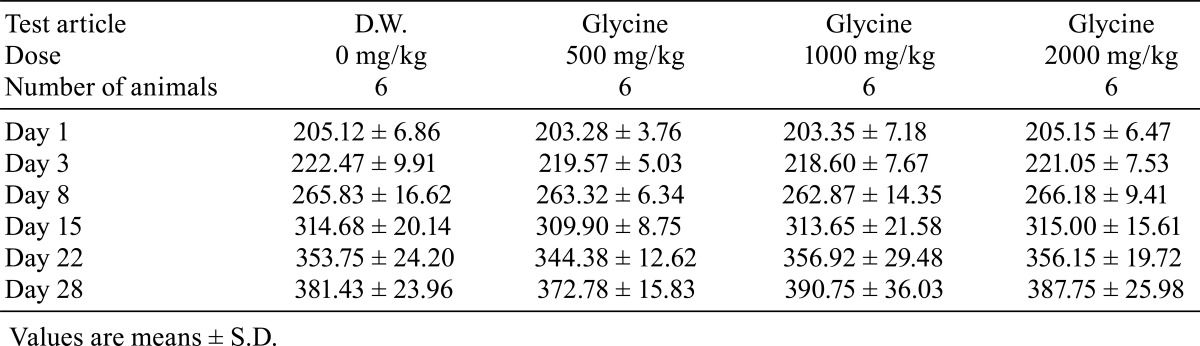
Table 2. Food Consumption (Unit: g).
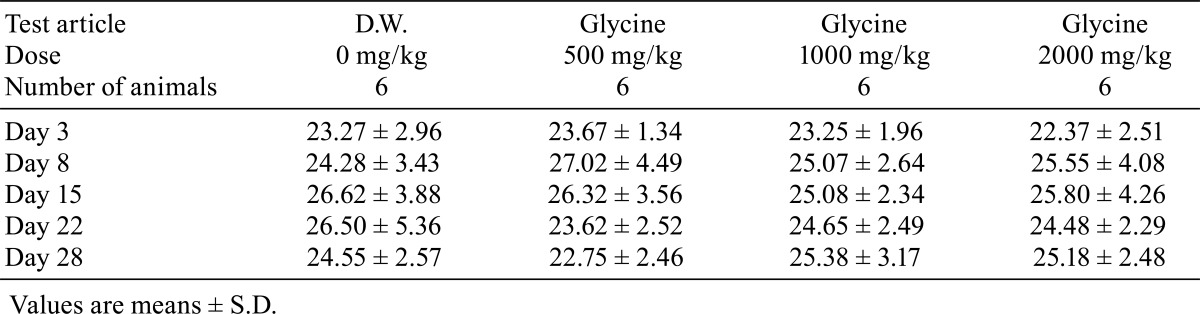
Table 3. Water Consumption (Unit: g).
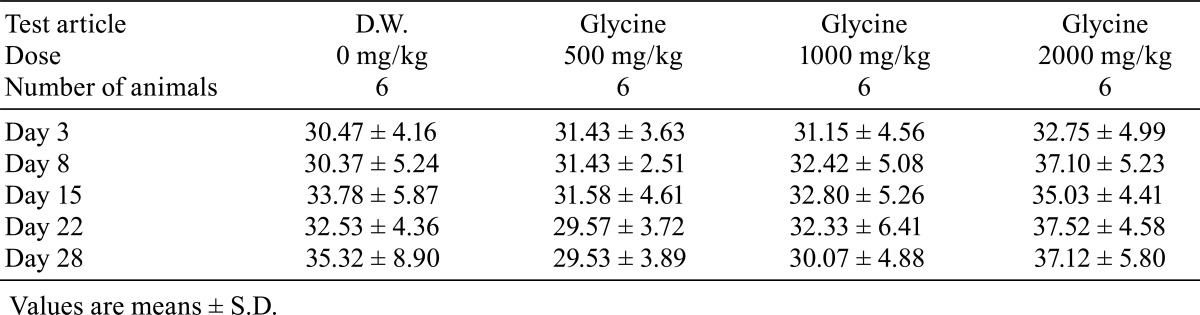
Daily urine volume and total amount of Cl excretion were significantly higher (p<0.05) in the 2000 mg/kg dose group, and urine pH and urinary protein showed lower trends in the glycine-treated groups. There were no changes in other urinary parameters (Table 4). There were no significant differences in hematology parameters between the glycine-treated groups and control group (Table 5). The phospholipids level was significantly higher (p<0.05) in the glycine 2000 mg/kg dose group than in the control group (Table 6). No other significant differences in blood chemistry between glycine-treated groups and the control group were found.
Table 4. Urinalysis.
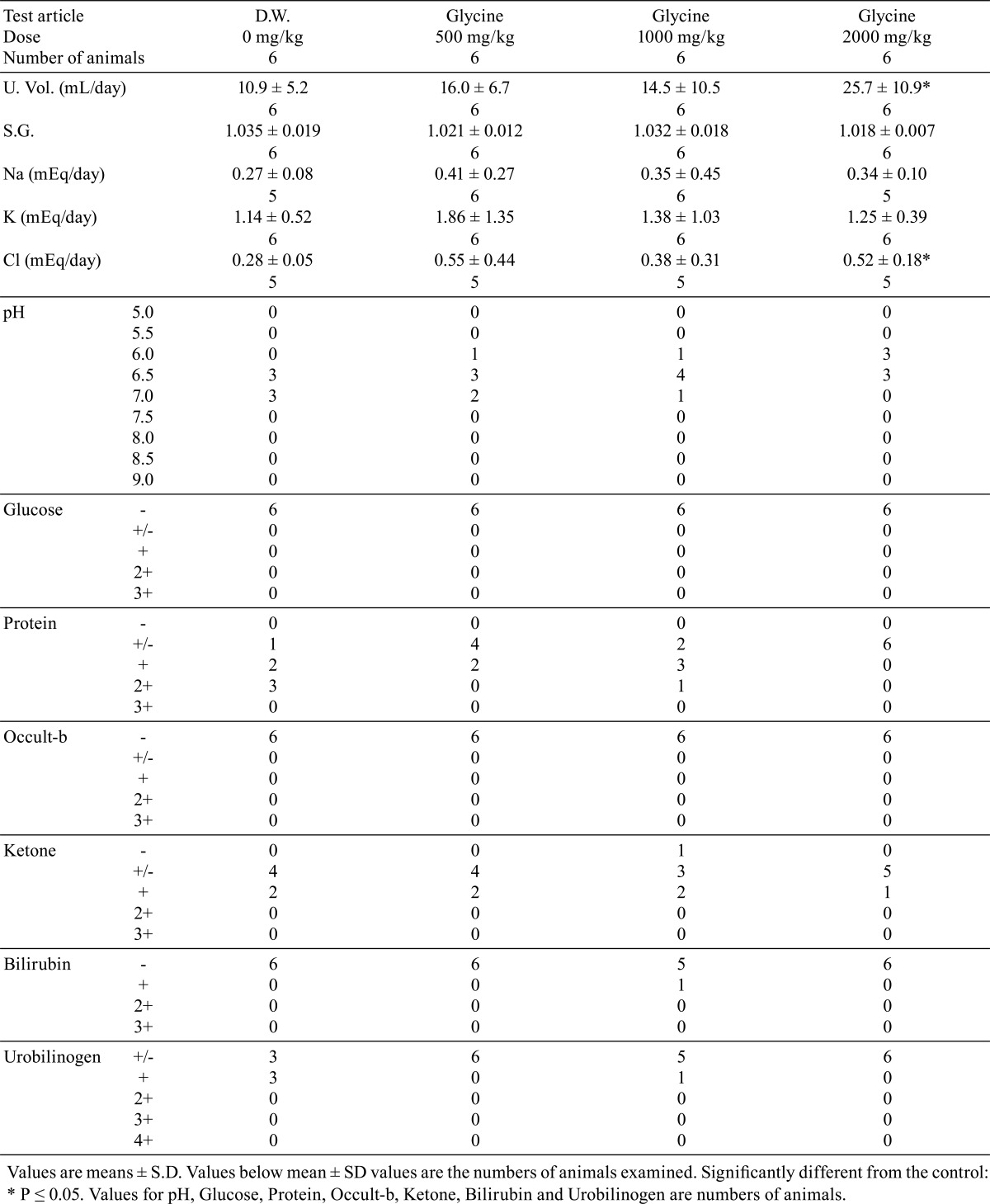
Table 5. Hematology.
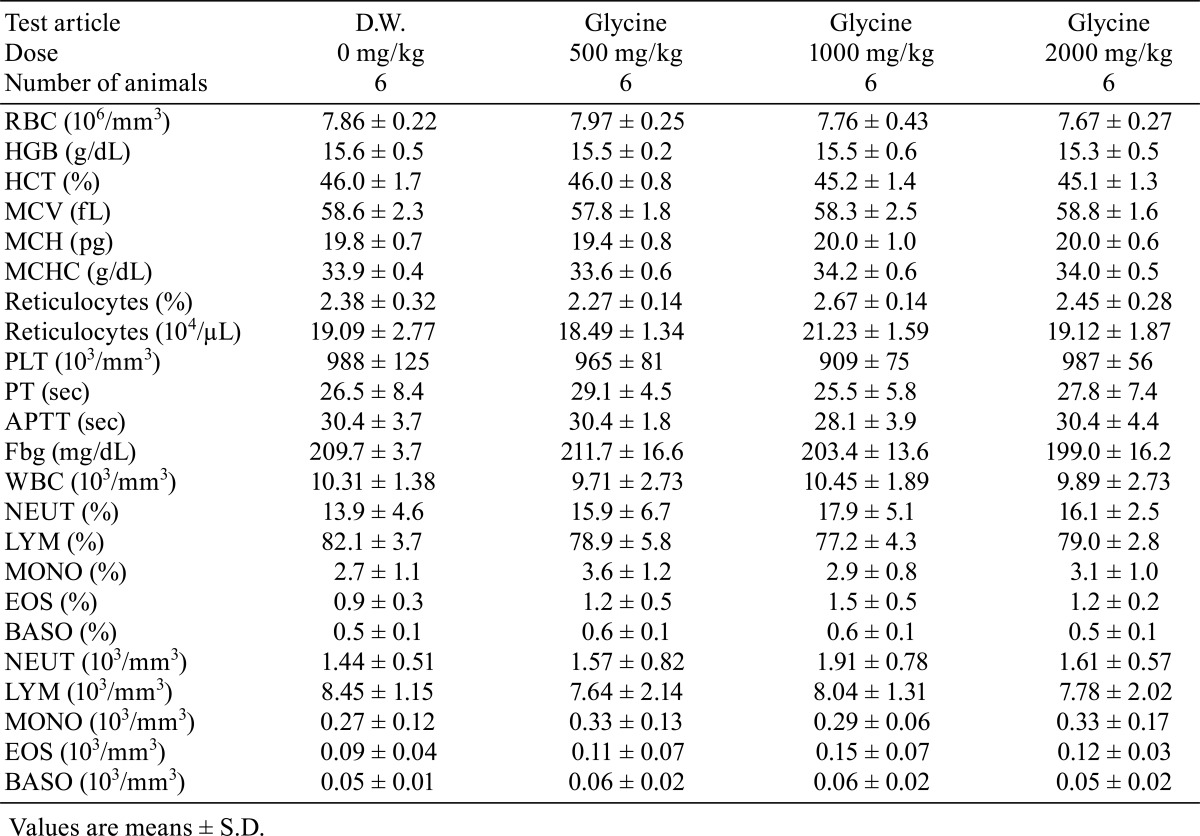
Table 6. Blood Chemistry.
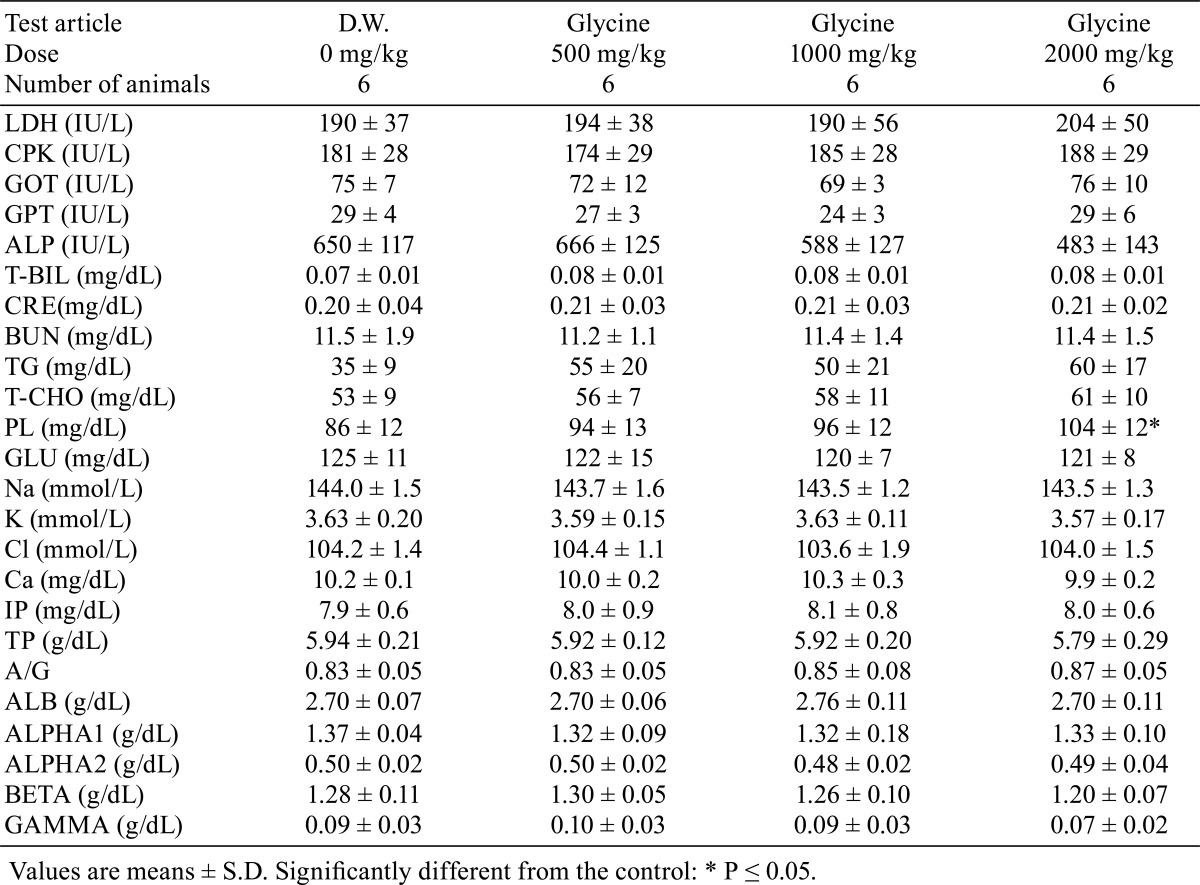
There were no significant differences in absolute or relative organ weights between the glycine-treated groups and control group (Tables 7 and 8). No treatment-related gross pathological findings were observed at the end of the administration period (Table 9). There were no treatment-related histopathological findings in any of the tissues and organs examined (Table 10). Microscopic findings noted in treated animals were considered incidental changes, as they also occurred in controls, were of low incidence, had no dose-dependency of incidence or severity and/or were common background findings with regard to the species of same strain, sex and age.
Table 7. Organ Weight.
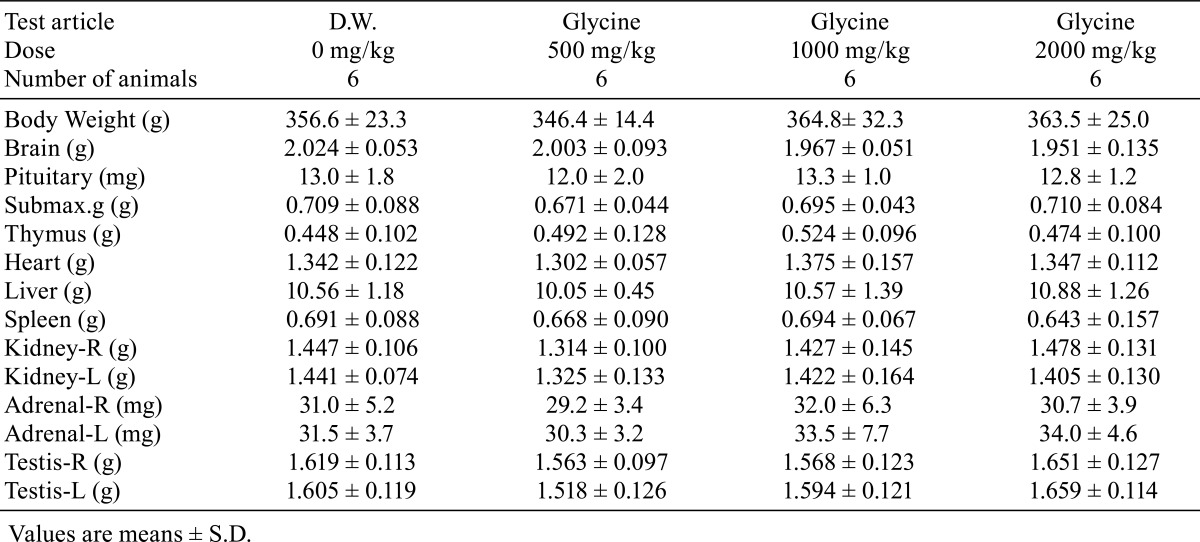
Table 8. Relative Organ Weight.
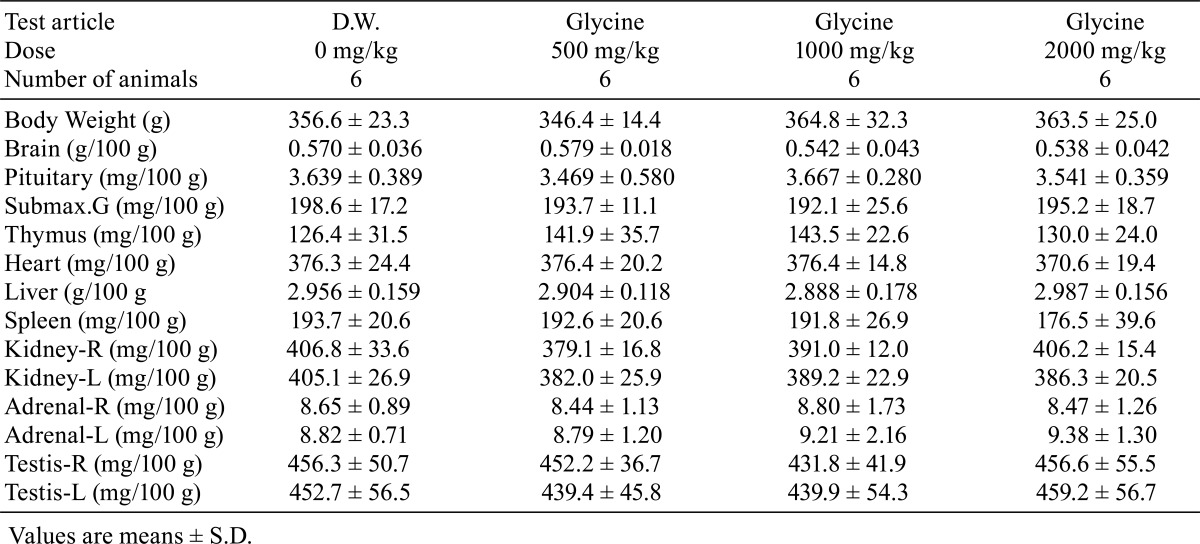
Table 9. Gross Pathological Findings.
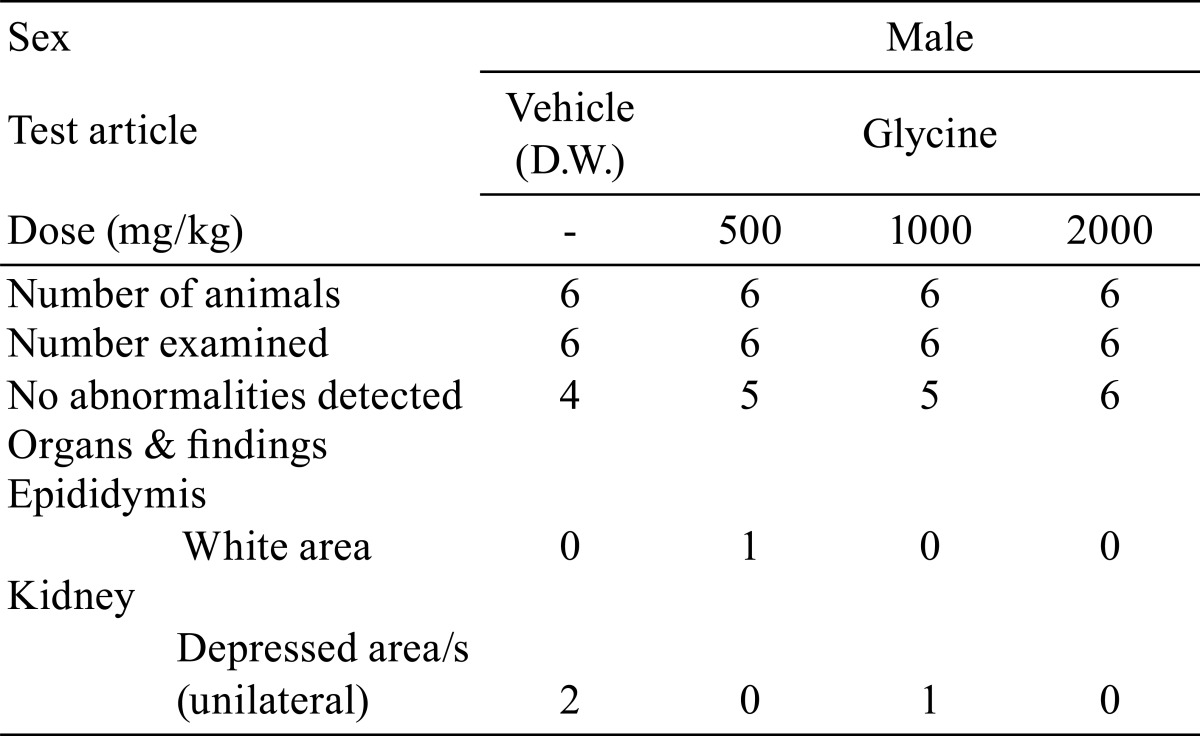
Table 10. Histopathological Findings.
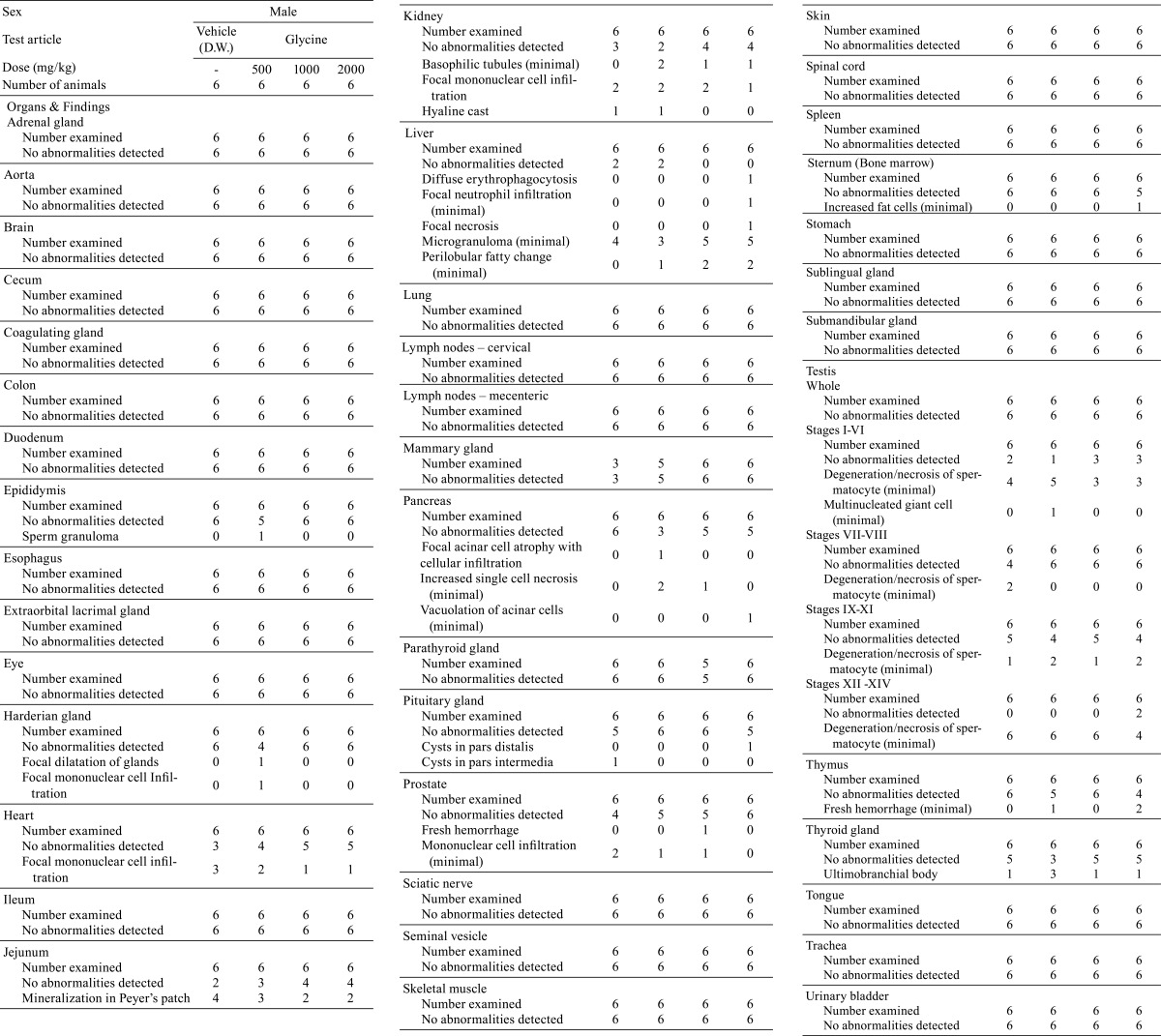
Discussion
In the present study on oral toxicity of glycine in rats, no deaths or treatment-related effects on clinical signs were seen during the 4-week administration period, and there were no significant changes in body weight, food consumption, water consumption or hematology that were attributed to the administration of glycine. Statistically significant increases in daily urinary volume and total amount of urinary Cl, and declining trends in urine pH and urinary protein in the glycine 2000 mg/kg dose group were considered to be of little toxicological significance, because there were no histopathological correlates in the kidneys and urinary bladder and no changes in kidney weight or other urinary parameters. The small, but statistically significant, increase in phospholipids in blood chemistry in the 2000 mg/kg dose group was not considered to be toxicologically significant. There were no treatment-related changes in organ weights, gross pathological or histopathological findings at any dose examined.
In a previous 2-year carcinogenicity study of glycine in rats by drinking-water administration4, decreased CPK, necrosis in the renal papillae, papillomas in the renal pelvis and several other changes were reported. However, in the present study, we did not observe decreased CPK or glycine-related histopathological changes in the kidney. In contrast, we observed an increased volume of urine and total amount of urinary Cl and decreased trends of urine pH and urinary protein in the glycine-treated groups in our study, though these changes were not reported in the 2-year carcinogenicity study. The differences in these results are probably mainly attributable to differences in the experimental design, including the duration and/or the administration method. Still, we would like to point out that there is a possibility that some changes related to these alterations in urine properties may have led to the necrosis in the renal papillae and/or papillomas in the renal pelvis. However, the relationships between these changes remain to be determined.
In conclusion, once-daily oral administration of glycine at 500, 1000, or 2000 mg/kg/day in a volume of 10 mL/kg for 4 weeks to male SD rats (Crl: CD (SD)) caused no toxicologically significant change in the animals. The no-observed-adverse-effect level of glycine is at least 2000 mg/kg under the conditions of this study.
Conflict of Interest: We certify that there is no actual or potential conflict of interest in relation to this article.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Anderson SA, and Raiten DJ. Safety of amino acids used as dietary supplements. Life Sciences Research Office, Federation of American Societies for Experimental Biology. 167-171. 1992
- 2.Safety (MSDS) data for glycine. The Physical and Theoretical Chemistry Laboratory, Oxford University. 2005. Retrieved 2006-11-01.
- 3.European Food Safety Authority (EFSA) SCIENTIFIC OPINION Flavouring Group Evaluation 79, (FGE.79). Consideration of amino acids and related substances evaluated by JECFA (63rd meeting) structurally related to amino acids from chemical group 34 evaluated by EFSA in FGE.26Rev1(2008). The EFSA Journal. 870: 1-46. 2008.
- 4.Kitahori Y, Konishi N, Hayashi I, Nakahashi K, Kitamura M, Nakamura Y, Matsuda H, Fukushima Y, Yoshioka N, and Hiasa Y. Carcinogenicity study of glycine in Fischer 344 rats. J Toxicol Pathol. 7: 471–480 1994. [Google Scholar]
- 5.Partial Revision of Guidelines for Repeated-dose Toxicity Studies. Notification No. 655 of the Pharmaceutical and Medical Safety Bureau. Ministry of Health and Welfare, Japan. 1999 [Google Scholar]


