Abstract
Background
Standardization of surgical and pathologic techniques is crucial to the interpretation of studies evaluating adjuvant therapies for pancreatic cancer (PC).
Methods
To assess the degree to which treatment administered prior to enrollment of patients in trials of adjuvant therapy is quality controlled, the operative and pathology reports of patients in American College of Surgeons Oncology Group (ACOSOG) Z5031—a national trial of chemoradiation following pancreaticoduodenectomy (PD)—were rigorously evaluated. We analyzed variables with the potential to influence staging or outcome.
Results
80 patients reported to have undergone R0 (75%) or R1 (25%) pylorus-preserving (38%) or standard (62%) PD were evaluated. A search for metastases was documented in 96% of cases. The proximity of the tumor to the superior mesenteric vein was reported in 69%; vein resection was required in 9% and lateral venorrhaphy in 14%. The method of dissection along the superior mesenteric artery (SMA) was described in 68%, being ultrasonic dissection (17%), stapler (24%), and clamp and cut (59%). SMA skeletonization was described in 25%, and absence of disease following resection was documented in 24%. The surgeon reported marking the critical SMA margin in 25%; inking was documented in 65% of cases and evaluation of the SMA margin was reported in 47%. A range of 1–49 lymph nodes was evaluated. Only 34% of pathology reports met College of American Pathologists criteria.
Conclusions
Trials of adjuvant therapy following PD suffer from a lack of standardization and quality control prior to patient enrollment. These data suggest areas for improvement in the design of multidisciplinary treatment protocols.
Pancreaticoduodenectomy (PD) remains the cornerstone of therapy for localized cancers of the pancreatic head and uncinate process.1–3 Postoperative systemic chemotherapy and/or chemoradiation increases the overall survival rate among patients who undergo microscopically (R0) or macroscopically (R1) complete PD.4–8 Fundamental components of a multidisciplinary approach to resectable PC therefore include accurate preoperative disease staging, a meticulous surgical technique with attention to oncologically relevant soft tissue margins, and precise pathologic analysis of the PD specimen prior to the administration of adjuvant therapy.
In recognition of the potential influence of surgical technique on oncologic outcome and in an attempt to minimize variation in surgical practice, consensus guidelines for the perioperative management of patients with colorectal cancer have been established.9 Although recently published guidelines attempt to standardize the perioperative management of pancreatic cancer, these guidelines are broad and do not detail several critical technical aspects of PD.10 Moreover, although the College of American Pathologists (CAP) and the American Joint Committee on Cancer (AJCC) both have standard protocols in place to direct histopathologic analysis of PD specimens, these guidelines likewise lack detail, and the degree to which they are observed varies.11–13 Inconsistency therefore exists in surgical approaches to PD and in pathologic methods used to evaluate surgical specimens. These inconsistencies may adversely influence the accuracy of assessments of patients’ eligibility for clinical trials of adjuvant therapies, the interpretation of the results of such trials, and ultimately oncologic outcomes.
The two goals of this study are: (1) to identify and evaluate sources of variability in surgical technique and methods of pathologic review among patients with resectable PC prior to enrollment in multi-institutional clinical trials of postoperative adjuvant therapy, and (2) to examine the completeness of documentation of these variables in the operative and pathology reports generated for these patients. To these ends, we critically examined pre-enrollment clinical data of patients treated in the American College of Surgeons Oncology Group (ACOSOG) Z5031 trial.
PATIENTS AND METHODS
Patients and Treatments
Between November 2003 and December 2005, 80 evaluable patients were enrolled in ACOSOG Z5031. The trial was approved by the institutional review board of each participating institution, and all participants provided written informed consent.
Eligibility requirements have been previously described in detail.14 All patients had Eastern Cooperative Oncology Group performance status of 0 or 1 and histologically proven adenocarcinoma of the head or uncinate process of the pancreas following potentially curative PD. Central review of preoperative staging studies was not required. Since surgery was not part of the protocol, the method and technical aspects of PD were not standardized. All cancers were pathologically staged as T1–3, N0–1, M0.11 The methods of evaluating the surgical specimen were not dictated by the protocol.
Following a baseline radiographic evaluation, patients began therapy with external-beam radiation, 5-fluorouracil, cisplatin, and subcutaneous interferon-α followed by systemic 5-fluorouracil. Follow-up was scheduled to be performed until each patient's death.
Study Design
A panel of four pancreatic surgeons and a statistician compiled a list of factors associated with surgical management and histopathologic evaluation of the surgical specimen thought to vary in clinical practice and to potentially influence oncologic outcome, as determined by literature review and expert experience (Table 1). The operative and pathology reports of each patient were then meticulously scrutinized by two surgeons independently to evaluate the technical aspects of the surgical procedure and pathologic assessment performed prior to protocol enrollment. Documentation of these factors was also critically evaluated. Results for each reviewer were collected and compared. In the event of discordance between reviewers for a given factor, that factor was re-evaluated by the group and consensus obtained.
TABLE 1.
Clinical variables evaluated as part of the critical review of the operative and pathology reports of each patient enrolled in ACOSOG Z5031
| Operative characteristics and reporting |
| Author of operative report |
| Type of PD performed (standard, pylorus-preserving) |
| Estimated blood loss |
| Transfusion required |
| Search for extrapancreatic disease |
| Liver |
| Peritoneum |
| Peritoneal cytology sample obtained |
| Laparoscopy performed prior to laparotomy |
| Hepatic artery/GDA lymph node dissection |
| Relationship of tumor to SMV |
| Description of SMV dissection and resection if required |
| SMA skeletonization |
| Method of dissection of SMA margin |
| Macroscopically complete resection documented |
| Margins analyzed by frozen section |
| SMA |
| Pancreatic neck |
| CBD |
| Others |
| Surgeon–pathologist communication |
| Communication between surgeon and pathologist |
| PD specimen oriented by surgeon for pathologist |
| Margins identified/marked by surgeon |
| SMA |
| Pancreatic neck |
| CBD |
| Others |
| Pathologic characteristics and reporting |
| Report meets CAP criteria |
| Specimen inked |
| Margins evaluated by pathologist |
| SMA |
| Pancreatic neck |
| CBD |
| Others |
| Lymph nodes evaluated |
| Total evaluated |
| Total positive |
| Other pathologic information reported |
| Tumor size/extent |
| Tumor grade |
| Lymphovascular invasion |
| Perineural invasion |
| AJCC TNM stage designated and recorded |
PD pancreaticoduodenectomy, GDA gastroduodenal artery, SMV superior mesenteric vein, SMA superior mesenteric artery, CBD common bile duct, CAP College of American Pathologists, AJCC American Joint Commission on Cancer, TNM tumor–node–metastasis staging system
Operative Characteristics and Reporting
The analysis of the operative report focused on the surgeon's assessment and documentation of clinical stage and the techniques used to dissect relevant surgical margins during the critical steps of PD. The surgeon's documentation of the disease burden prior to, during, and following resection was carefully evaluated. The anatomic relationship of the primary tumor to the superior mesenteric vein (SMV) was determined from the surgeon's documentation of either this relationship or the noted difficulty with which the SMV was dissected from the primary tumor.
The superior mesenteric artery (SMA) was considered to have been skeletonized if the surgeon clearly described dissection along the lateral aspect of the SMA and visualization of the artery's adventitia. Such a description was perceived to indicate removal of the uncinate process and all associated soft tissue between the uncinate process and SMA. If the uncinate process was released from its retroperitoneal attachments without documented visualization of the SMA or if a stapler was used for this dissection, the SMA was considered incompletely skeletonized.
Pathologic Characteristics and Reporting
The review of the pathology report focused on the evaluation of surgical margins (on frozen and permanent sections) and lymph nodes, as well as the adequacy of reporting as standardized by the CAP.12 The CAP guidelines, recently updated in October 2009, define the elements regarded as essential components of the surgical pathology report for PC. These components include a description of the gross size and anatomic location of the primary tumor and a determination of tumor type and grade. Use of the tumor–node–metastasis (TNM) staging system described by the AJCC (seventh edition) is advocated.13 Both the CAP and the AJCC encourage the inclusion of a description of the microscopic status of the soft tissue margin along the right lateral border of the SMA (referred to in the 6th AJCC edition and CAP as the “retroperitoneal margin” and “uncinate process margin,” respectively, but now referred to as the “SMA margin” in the 7th AJCC edition), the pancreatic neck margin, and the common bile duct margin in all PD pathology reports.11,13,15 The CAP guidelines also advocate reporting the status of the posterior retroperitoneal (radial) margin, representing the posterior surface of the pancreas, and the AJCC recommends the inclusion of a description of the proximal and distal enteric margins. In this study, we focused on the SMA, pancreatic neck, and common bile duct margins, because the posterior surface of the pancreas is not generally considered a surgical transection margin and the enteric margins are rarely involved by cancer.
Although both the CAP and AJCC guidelines recommend a specific handling procedure for each margin (i.e., the examination of perpendicular cuts through the SMA margin, and en face sectioning of the pancreatic neck and common bile duct margins), the specific protocol utilized in each case was not always clear, despite a thorough review of the pathology report. Documentation of simple examination of each margin was therefore considered sufficient for the purpose of this analysis.
RESULTS
Eighty evaluable patients were enrolled in ACOSOG Z5031 by 15 institutions (median 3 patients/institution; range 2–13). All patients were reported to have undergone potentially curative, grossly complete (R0, 60 patients, 75%; or R1, 20 patients, 25%) PD. A pylorus-preserving procedure was performed in 30 cases (38%). Following resection, tumors were pathologically staged as IA (4 cases, 5%), IB (2 cases, 3%), IIA (16 cases, 20%) or IIB (58 cases, 73%).
The operative report was dictated by the attending surgeon in 64 cases (80%). The presence or absence of a visible mass in the pancreatic head as demonstrated on pretreatment imaging studies was documented in 61 operative reports (76%). However, an explicit assessment of clinical stage that included documentation of both the relationship of the primary tumor to the mesenteric vessels and the absence of metastatic disease was identified in only ten (13%). Estimated operative blood loss was reported in 49 cases (61%); the need (or lack of need) for blood transfusion was documented in 14 (18%).
An intraoperative search for extrapancreatic disease was reported in 77 cases (96%), but a specific description of the liver and peritoneum was provided in only 64 (80%) and 54 (68%), respectively. The proximity of the primary tumor to the SMV was explicitly documented in only 55 operative reports (69%), but careful review of each report enabled an assessment of this tumor–vessel relationship in all cases. The primary tumor clearly did not abut or involve the SMV in 44 cases (55%). In 18 patients (23%), the primary tumor appeared to the surgeon to involve the lateral wall of the SMV: 7 of these patients underwent segmental resection of the SMV; the remainder underwent resection and repair of a segment of the lateral vein wall. The primary tumor of another 18 patients (23%) approximated the SMV but was dissected away from the vein with documented difficulty.
Of 54 cases (68%) in which the surgical technique used to dissect the uncinate process of the pancreas from the retroperitoneum was well-documented, a clamp and cut technique was used in 32 (59%), an ultrasonic dissector was used in 9 (17%), and a vascular stapler was used in 13 (24%). Skeletonization of the SMA was clearly documented in the operative reports of only 20 patients (25%), while review of the operative note revealed a lack of documented attention to the SMA in 47 (59%). In 11 cases (15%), whether or not the SMA had been completely skeletonized could not be determined, and this portion of the operative note was missing in 1. A statement documenting the absence of residual disease after removal of the PD specimen was made in only 19 reports (24%).
The pancreatic neck margin was designated by the surgeon (marked or sent as a separate specimen) in 60 cases (75%). Frozen-section analysis of this margin was performed in 64 cases (80%). The common bile duct margin was identified by the surgeon in 47 cases (59%) and analyzed with a frozen section in 51 (64%). The SMA margin was marked for the pathologist in 20 cases (25%) and analyzed with frozen sections in 10 (13%). Communication between the surgeon and pathologist regarding specimen orientation was documented in only 12 cases (15%).
Of 79 complete surgical pathology reports available for review, 74 (94%) documented the maximum tumor diameter. Tumor subtype and grade were reported for all patients. Inking of the specimen was documented in 52 cases (66%).
The frequencies of microscopic assessment of the SMA, pancreatic neck, and common bile duct margins are summarized in Table 2, along with the results of those assessments. Of significant concern, clear evidence that the critical SMA margin had been evaluated was identified in only 37 reports (47%). Furthermore, for only 36 specimens (46%) was microscopic examination of all three margins performed as mandated by the AJCC.11,13
TABLE 2.
Frequency with which surgical margins were evaluated prior to enrollment in ACOSOG Z5031 as determined by critical review of the pathology reports (n = 79)
| Result | No. of patients (%) |
|||
|---|---|---|---|---|
| SMA | CBD | Panc | AJCCa,b | |
| Evaluated, n (%) | 37 (47) | 74 (94) | 79 (100) | 36 (46) |
| Positive, n (%) | 14 (38) | 2 (3) | 12 (15) | 16 (44) |
| Negative, n (%) | 23 (62) | 72 (97) | 67 (85) | 20 (56) |
SMA superior mesenteric artery margin, CBD common bile duct margin, Panc pancreatic neck margin, AJCC American Joint Committee on Cancer
One pathology report was incomplete at the time of review
Cases in which all three margins recommended by the AJCC (sixth edition)—SMA, CBD, and Panc—were evaluated11
Positive indicates at least one margin of the three AJCC margins was positive; negative indicates all three margins were negative
Examination of regional lymph nodes was described in all patients; a median of 12 (range 1–49) lymph nodes were examined per specimen, and 58 specimens (73%) were identified as containing positive lymph nodes. The AJCC TNM stage was reported in 39 cases (49%). Among 79 complete pathology reports examined, only 27 (34%) met basic criteria described in the CAP guidelines.12
DISCUSSION
Administration of postoperative therapy is essential to providing optimal outcomes for PC patients who undergo R0 or R1 PD. Of equal importance, however, are the components of care delivered prior to adjuvant therapy. Precise clinical staging, appropriate patient selection for surgery, meticulous surgical dissection, and accurate pathologic examination of the surgical specimen all enable assessment of and, perhaps more importantly, modulate the risk for disease recurrence following therapy. Unfortunately, these components of care are typically performed prior to protocol enrollment and are therefore rarely subject to the same degree of scrutiny and standardization as the adjuvant treatments that follow. Thus, paradoxically, the primary component of multimodality therapy protocols—indeed, the only component proven to be potentially curative—is not quality controlled in any way. In this study, we confirmed an absence of standardization and quality control of these factors by rigorous review of the primary data sources of patients enrolled in a national trial of adjuvant therapy.
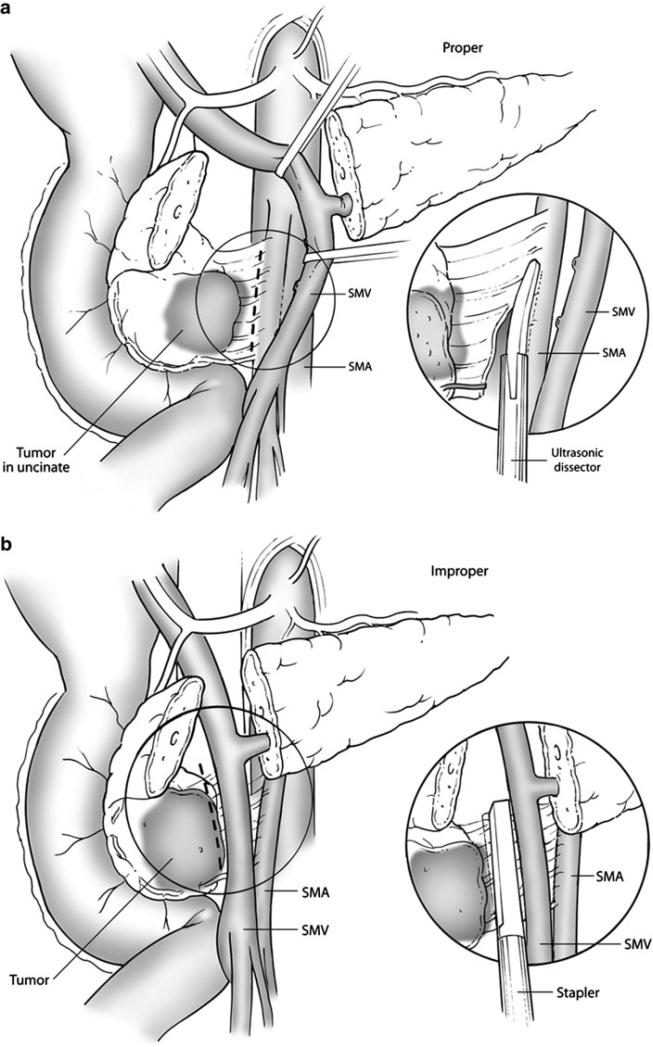
Variation in surgical quality has been demonstrated to influence rates of locoregional recurrence and long-term survival among patients with colorectal cancer.16–19 Such studies often focus on case volume as a surrogate marker of quality, but this metric is itself a surrogate marker of several technical variables.19,20 For patients with PC, one of the most important of these is the ability of the surgeon to achieve margin-negative resection. Rates of microscopically positive margins among PD specimens range between 16% and 85%, and margin status has been identified as an independent prognostic factor for survival in most series.1,2,15,21–24 As confirmed by this study, the soft tissue margin adjacent to the SMA is the most frequently positive margin after PD (Table 2).15 It has been suggested that microscopically incomplete resection at this margin is an exclusive reflection of aggressive tumor biology.5,6 However, given the fact that the medial edge of the tumor is often just millimeters away from the SMA, it stands to reason that medial retraction of the SMV and skeletonization of the lateral aspect of the SMA in thoroughly staged patients with potentially resectable disease can minimize rates of R1 resection (Fig. 1a).25
FIG. 1.
a Lateral retraction of the SMV and dissection along the adventitia of the SMA may enhance the potential for margin-negative resection; this technique is considered optimal. b Stapler dissection of the SMA margin and/or failure to retract the SMV laterally prior to skeletonization of the SMA may increase the potential for microscopically incomplete resection and subsequent local recurrence
Despite the oncologic significance of the SMA margin and the importance of technical factors in maximizing the R0 resection rate, we found wide variability in the techniques used for SMA dissection in patients enrolled in ACOSOG Z5031. Indeed, a stapler was used to free the uncinate process from the retroperitoneum in 24% of patients in whom the technique of dissection was documented. Although efficient, use of a stapler may leave as much as 43% of the soft tissue between the uncinate process and the SMA in situ and may therefore increase the potential for R1 resection.26,27 Furthermore, irrespective of the technique used for SMA dissection, adequate skeletonization of the SMA was documented in only 25% of patients in this study, and the SMA margin was marked or inked by the surgeon in only 25%. Together, these findings demonstrate a lack of emphasis placed upon this crucial step of PD and illustrate one potential source of the wide variability of margin-positivity rates seen in published reports. It is also conceivable, or even likely, that suboptimal surgical technique contributed to the high local recurrence rate (46%) observed in ACOSOG Z5031.14
Although the method with which pathologic examination of the PD specimen is performed is also critical, a universally accepted approach to the analysis of the PD specimen is lacking.11,12,23,28–32 As affirmed in this study, a major source of inconsistency is the assessment, description, and documentation of the oncologic status of the retroperitoneal tissues. We found documentation of the “uncinate process margin,” “retroperitoneal margin,” “SMA margin,” “radial margin,” “deep margin,” and “posterior margin”—all referring to margins in the retroperitoneum. Even the AJCC and CAP guidelines appear to disagree on the designation and relative importance of tissues in this region. The AJCC places emphasis on the margin of divided tissue just lateral to the SMA (the descriptor for which has been changed to the “SMA margin” to differentiate it from the tissue between the posterior surface of the uncinate process and vena cava). In contrast, until October 2009 the CAP emphasized the oncologic significance of the deep radial posterior surface of the pancreatic head, and the newly updated guideliness still refer to the SMA margin tissue as the uncinate margin.12,13,15 Perhaps as a result of this inconsistency and ambiguity, the status of the tissue margin adjacent to the SMA was documented in only 47% of patients enrolled in this trial. This, combined with diversity among pathologists with regard to the methods used to ink, section, and analyze the SMA and other margins, also helps to explain the disparity in published rates of R1 resection.11,12,23,30–32 Significantly, although only 25% of patients enrolled in ACOSOG Z5031 were reported to have undergone R1 resection, we found at least one positive margin in 44% of the 36 patients in whom evaluation of all three AJCC-recommended margins was documented.
The importance of accurate perioperative staging must also be emphasized as a means both to predict prognosis and to accurately exclude or enroll patients in adjuvant therapy trials. Not surprisingly, we identified wide variation in the perioperative staging methods used to evaluate patients, along with deficiency in reporting of key staging indicators (Table 3). Although preoperative computed tomography scan was performed on all patients, the quality of these scans and the accuracy of their interpretation were not subject to central review, and a basic description of preoperative disease stage based on these scans was documented in a minority of operative reports. Furthermore, we found a description of a systematic intraoperative search for extrapancreatic disease absent in a surprising number of cases. Moreover, less than one-quarter of operative reports documented the absence of macroscopic residual disease after removal of the surgical specimen. Combined, these findings suggest further potential for clinical heterogeneity among patients enrolled in trials of postoperative therapy for PC.
TABLE 3.
Frequency with which critical surgical and pathologic factors were documented in operative and pathology reports of patients enrolled on ACOSOG Z5031
| Clinical factor | Reported, n (%) |
|---|---|
| Surgical factorsa | |
| Type of resection | 80 (100) |
| Preoperative clinical stage | 10 (13) |
| Transfusion requirement | 14 (18) |
| Search for extrapancreatic disease | 77 (96) |
| Description of liver | 64 (80) |
| Description of peritoneum | 54 (68) |
| Relationship of tumor to SMV | 55 (69) |
| Technique of SMA dissection | 54 (68) |
| Marking of SMA marginb | 20 (25) |
| Absence of residual macroscopic disease | 19 (24) |
| Communication with pathologistb | 12 (15) |
| Pathologic factors | |
| Histologic subtype | 79 (100) |
| Inking performed | 52 (66) |
| Evaluation of SMA margin | 37 (47) |
| Examination of regional lymph nodes | 79 (100) |
| Maximum tumor diameter | 74 (94) |
| Tumor grade | 79 (100) |
| Lymphovascular invasion | 63 (80) |
| Perineural invasion | 75 (95) |
| AJCC TNM stage | 39 (49) |
| CAP guidelines observed | 27 (34) |
Operative dictation was performed by the attending surgeon in only 64 (80%) cases
Determined by simultaneous review of both operative and pathology reports
Like any retrospective study, ours is not without limitations. Clearly, our appraisal of the technical aspects of the staging, surgical, and pathologic methods utilized prior to enrollment in ACOSOG Z5031 is limited by the accuracy and comprehensiveness of the operative and pathology reports generated for these patients. It is possible that the surgical and pathologic documentation may have mis- or underrepresented clinical events or processes, particularly given the deficiencies identified in these reports (Table 3). However, all documents were authored and/or approved by the attending physician, minimizing this possibility. We further acknowledge that the retrospective evaluation of clinical events described in these reports is subject to interpretation by those gathering the data. We addressed this by requiring all data to be collected by two independent surgeons, with subsequent analysis by the complete panel of four surgeons. Indeed, a major strength of the analysis reported herein is the rigorous evaluation of primary data sources by experienced pancreatic surgeons as opposed to review of a large database by a trainee.
In summary, we found an overwhelming absence of consistency in perioperative staging, surgical treatment, and pathologic analysis for PC patients in this study. Although the independent influences of these factors and their documentation upon outcome are unknown, the overall effect could clearly result in heterogeneity among patients enrolled in clinical trials of adjuvant therapies, the potential for misinterpretation of the results of these trials, and unsatisfactory outcomes. Our data suggest critical areas for improvement in the design of multidisciplinary treatment protocols for patients with resectable PC.
ACKNOWLEDGMENT
Supported by funding from the US National Institutes of Health to the American College of Surgeons Oncology Group, grant U10 CA 76001.
This study was conducted for the American College of Surgeons Oncology Group.
Footnotes
Presented at the 63rd Annual Cancer Symposium of the Society of Surgical Oncology, St. Louis, March, 2010.
REFERENCES
- 1.Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–45. doi: 10.1016/j.gassur.2006.09.008. discussion 1345–6. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [16 August 2010];Pancreatic adenocarcinoma V.2.2010. NCCN Clinical Practice Guidelines in Oncology. 2009 Available at: http://www.nccn.org/professionals/physicians_gls/PDF/pancreatic.pdf.
- 4.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–68. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–96. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Dixon E. AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725–6. doi: 10.1245/s10434-009-0409-5. [DOI] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer . Exocrine pancreas. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. Springer; Chicago: 2002. pp. 157–64. [Google Scholar]
- 12.College of American Pathologists Cancer Protocols [16 August 2010];Pancreas (exocrine) 2009 Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/PancreasExo_09protocol.pdf.
- 13.American Joint Committee on Cancer . Exocrine pancreas. In: Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. Springer; Chicago: 2009. pp. 241–9. [Google Scholar]
- 14.Picozzi VJ, Abrams RA, Decker PA, Traverso LW, O'Reilly EM, Greeno E, et al. Multicenter, phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2010 doi: 10.1093/annonc/mdq384. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following ‘curative’ surgery for large bowel cancer: I. The overall picture. Br J Surg. 1984;71:12–6. doi: 10.1002/bjs.1800710813. [DOI] [PubMed] [Google Scholar]
- 17.Holm T, Johansson H, Cedermark B, Ekelund G, Rutqvist LE. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg. 1997;84:657–63. [PubMed] [Google Scholar]
- 18.Kockerling F, Reymond MA, Altendorf-Hofmann A, Dworak O, Hohenberger W. Influence of surgery on metachronous distant metastases and survival in rectal cancer. J Clin Oncol. 1998;16:324–9. doi: 10.1200/JCO.1998.16.1.324. [DOI] [PubMed] [Google Scholar]
- 19.Stocchi L, Nelson H, Sargent DJ, O'Connell MJ, Tepper JE, Krook JE, et al. Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol. 2001;19:3895–902. doi: 10.1200/JCO.2001.19.18.3895. [DOI] [PubMed] [Google Scholar]
- 20.Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, Jolley D. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin. 2009;59:192–211. doi: 10.3322/caac.20018. [DOI] [PubMed] [Google Scholar]
- 21.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–58. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. (discussion 1210–1) [DOI] [PubMed] [Google Scholar]
- 23.Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford) 2009;11:18–24. doi: 10.1111/j.1477-2574.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz MH, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58:111–25. doi: 10.3322/CA.2007.0012. [DOI] [PubMed] [Google Scholar]
- 25.Evans DB, Hess KR, Pisters PW. ESPAC-1 trial of adjuvant therapy for resectable adenocarcinoma of the pancreas. Ann Surg. 2002;236:694. doi: 10.1097/01.SLA.0000037256.09376.FC. (author reply 694–6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baque P, Iannelli A, Delotte J, de Peretti F, Bourgeon A. Division of the right posterior attachments of the head of the pancreas with a linear stapler during pancreaticoduodenectomy: vascular and oncological considerations based on an anatomical cadaver-based study. Surg Radiol Anat. 2009;31:13–7. doi: 10.1007/s00276-008-0353-2. [DOI] [PubMed] [Google Scholar]
- 27.Evans DB, Pisters PW. Novel applications of endo GIA linear staplers during pancreaticoduodenectomy and total pancreatectomy. Am J Surg. 2003;185:606–7. doi: 10.1016/s0002-9610(02)01400-9. [DOI] [PubMed] [Google Scholar]
- 28.Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–80. doi: 10.1097/00006676-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Chatelain D, Flejou JF. [Pancreatectomy for adenocarcinoma: prognostic factors, recommendations for pathological reports]. Ann Pathol. 2002;22:422–31. [PubMed] [Google Scholar]
- 30.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–6. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]
- 31.Khalifa MA, Maksymov V, Rowsell C. Retroperitoneal margin of the pancreaticoduodenectomy specimen: anatomic mapping for the surgical pathologist. Virchows Arch. 2009;454:125–31. doi: 10.1007/s00428-008-0711-9. [DOI] [PubMed] [Google Scholar]
- 32.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–7. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]