Figure 2.
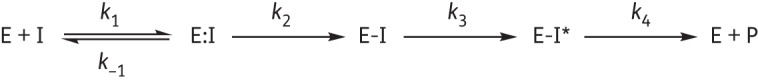
Interactions of FOX-4 (E) with inhibitors (I) are depicted by this reaction scheme. The formation of the Michaelis complex E:I is represented by the dissociation constant K = k−1/k1. k2 is the first-order rate constant for acylation or formation of E-I. The appearance of chromophores (E-I*), as suggested by UVD spectroscopy, is represented by the rate constant k3. Finally, product formation (E + P) as revealed by tn >1 for the majority of the compounds tested is denoted by the rate constant k4.
