Abstract
Objective:
To assess the technical feasibility, safety and clinical outcome of CT-guided high-dose-rate brachytherapy (CT-HDRBT) for achieving local tumour control (LTC) in isolated lymph node metastases.
Methods:
From January 2008 to December 2011, 10 patients (six males and four females) with isolated nodal metastases were treated with CT-HDRBT. Five lymph node metastases were para-aortic, three were at the liver hilum, one at the coeliac trunk and one was a left iliac nodal metastasis. The mean lesion diameter was 36.5 mm (range 12.0–67.0 mm). Patients were followed up by either contrast-enhanced CT or MRI 6 weeks and then every 3 months after the end of treatment. The primary end point was LTC. Secondary end points included primary technical effectiveness rate, adverse events and progression-free survival.
Results:
The first follow-up examination after 6 weeks revealed complete coverage of all nodal metastases treated. There was no peri-interventional mortality or major complications. The mean follow-up period was 13.2 months (range 4–20 months). 2 out of 10 patients (20%) showed local tumour progression 9 and 10 months after ablation. 5 out of 10 patients (50%) showed systemic tumour progression. The mean progression-free interval was 9.2 months (range 2–20 months).
Conclusion:
CT-HDRBT is a safe and effective technique for minimally invasive ablation of nodal metastases.
Advances in knowledge:
CT-HDRBT of lymph node metastases is feasible and safe. CT-HDRBT might be a viable therapeutic alternative to obtain LTC in selected patients with isolated lymph node metastases.
Metastatic spread to lymph nodes is a common event in the course of many thoracic and abdominal malignancies and has considerable clinical implications [1]. The management of metastatic disease is complex, and treatment modalities reported in the literature are heterogeneous, depending largely on the localisation of metastatic nodes as well as the patient’s performance status and treatment history. Hence, the optimal management for patients with isolated lymph node metastasis has not yet been established.
Although the results of several studies suggest a potential benefit to the cytoreduction of isolated nodal disease among patients with ovarian, hepatocellular, renal or colorectal carcinoma, data on the role of repeated surgical resection of confined lymph node metastases are limited, and response rates of nodal metastases to chemotherapy are inconsistent [2–7].
Over the past decades, interventional oncology has expanded its role, and minimally invasive tumour ablation techniques have become a cornerstone in the multimodal treatment of oncological patients. The clinical success of thermal ablation techniques, such as radiofrequency ablation (RFA), has generated a large body of literature on the treatment of liver and lung tumours, while data on its use for the treatment of lymph node metastases remain scarce. CT-guided high-dose-rate brachytherapy (CT-HDRBT) is a radio-ablative technique that was established about 10 years ago to overcome the limitations of thermal ablative techniques [8]. Features such as high accuracy in dose distribution and applicability regardless of tumour diameter and location have contributed to the attractiveness of this technique. In recent years, several studies have reported encouraging results for the treatment of lung and liver tumours as well as extrahepatic and extrapulmonary malignancies [9–12]. The purpose of the present study is to report the results of CT-HDRBT for achieving local tumour control (LTC) in the treatment of isolated lymph node metastases.
MATERIALS AND METHODS
This retrospective analysis includes all consecutive patients with isolated lymph node metastases treated by CT-HDRBT at our institution between January 2008 and December 2011. All patients had isolated recurrent nodal metastases from different solid cancers as diagnosed by contrast-enhanced CT or MRI. The suitability of each patient for local ablation was discussed at an interdisciplinary tumour board to ensure that all suitable treatment alternatives had been considered. Patients were considered for CT-HDRBT in the case of isolated, medically or technically non-resectable nodal metastases accessible for percutaneous ablation. To undergo the procedure, patients had to have a platelet count of >50 000 μl, a prothrombin time of <50 s and a partial thromboplastin time of <50 s. If indicated, the haemostatic function was corrected (e.g. platelet concentration). This study was approved by the institutional review board. Written informed consent was obtained from each patient before the procedure.
Interventional technique
The interventional technique has been described in detail elsewhere [10]. Briefly, it consists of two major steps: (1) CT-guided catheter placement and (2) high-dose-rate irradiation in the afterloading technique. Catheter implantation was completed under CT-fluoroscopic guidance after analgesia and sedation (midazolam and fentanyl) and local anaesthesia (lidocaine) of the puncture site. Following these preparations, a 17-gauge needle was advanced to the target lesion for inserting a stiff angiographic guidewire (Amplatz™; Boston Scientific, Boston, MA). Subsequently, the needle was removed and replaced by a flexible 6-French sheath (Radifocus®; Terumo, Tokyo, Japan). Finally, the angiographic guidewire was removed and a 6-French afterloading catheter (Primed™; Halberstadt Medizintechnik GmbH, Halberstadt, Germany) was inserted through the sheath into the tumour. After placement of the afterloading catheter, a contrast-enhanced scan of the abdomen was obtained to verify correct catheter positioning and to plan treatment. Computer-based three-dimensional treatment planning was performed on a dedicated workstation using the acquired data set and Brachyvision™ software (Gammamed™; Varian, Palo Alto, CA). Finally, all afterloading catheters were digitised from the tip to the body exit point. Subsequently, the clinical target volume (CTV) of each tumour and all at-risk structures (e.g. stomach, bowel, spinal cord, hepatic hilus) were outlined (Figure 1). Based on this information, the Brachyvision software automatically supplied an irradiation plan for the indicated target volume. If necessary, dwell points and dwell times for the iridium-192 source inside the afterloading catheters were optimised manually to ensure full coverage of the target volume while simultaneously sparing the at-risk structures. All metastases were treated by single-fraction irradiation using the afterloading technique using an iridium-192 radiation source with a nominal activity of 10 Ci. The minimum dose to cover the CTV was 15 Gy. Maximum doses of >50 Gy were allowed within the tumour centre. After irradiation, the afterloading catheters were carefully removed and the puncture channels were sealed with thrombogenic material (Gelfoam®; Pfizer Inc., New York, NY) to avoid bleeding.
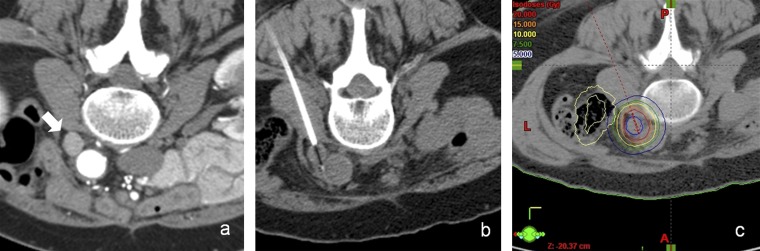
Figure 1.
(a) Baseline CT scan in the prone position shows a para-aortic nodal metastasis (arrow). (b) CT-guided positioning of the afterloading catheter in the nodal metastasis. (c) Planning CT after CT-guided positioning of the afterloading catheter. Visible tumour borders were defined as the clinical target volume. Dose distribution was adjusted. The prescribed minimum tumour-surrounding dose was 20 Gy.
Follow-up and evaluation criteria
Patients were followed up by either contrast-enhanced CT or MRI 6 weeks and then every 3 months after the end of the treatment. The primary end point was LTC. Secondary end points included adverse events and progression-free survival (PFS). Any increase in the largest lesion diameter compared with baseline at any time during the follow-up was defined as local tumour progression (LTP). PFS was defined as LTC after CT-HDRBT without tumour progression elsewhere. LTP rates, time to progression and complications were documented. The major and minor complications were defined according to the Society of Interventional Radiology reporting standards [13].
Statistical analysis
Medical records and imaging reports were entered into a worksheet for storage (Excel 2002 v. 10; Microsoft©, Redmond, WA) and subsequently imported into a statistical software package (SPSS® v. 18.0; SPSS, Chicago, IL) for analysis. All quantitative data are expressed as the mean ± range, unless otherwise indicated.
RESULTS
During the above-mentioned study interval, a total of 10 patients with isolated nodal metastases were treated with CT-HDRBT. There were six males and four females, with a mean age of 63.6 years (range 47–75 years). The primary tumour was hepatocellular carcinoma (HCC), colorectal carcinoma and renal cell carcinoma in two patients each. One patient each had intrahepatic cholangiocarcinoma, gastric cancer, neuroendocrine tumour and concomitant HCC and renal cell carcinoma. All patients included in the study had undergone previous therapies (including chemotherapy, surgery and/or interventional radiology procedures) for the primary tumour. Five lymph node metastases were para-aortic, three were at the liver hilum, one at the coeliac trunk and one was a left iliac nodal metastasis. The mean lesion diameter was 36.5 mm (range 12.0–67.0 mm). The average minimum tumour-enclosing dose was 16.8 Gy (15.0–20.0 Gy). Three patients were treated with a tumour-enclosing dose of 20 Gy, and seven with a lower minimum dose of 15 Gy to reduce exposure of adjacent high-risk structures. The mean CTV was 47.6 ml (2.4–120.3 ml). The mean coverage of the CTV was 96.1% (69.9–100%). There was no peri-interventional mortality or major complications. The mean follow-up period was 13.2 months (range 4–20 months). 2 of the 10 patients showed a LTP 9 and 10 months after ablation. Owing to concomitant systemic tumour progression, both patients were not amenable to repeated CT-HDRBT and were scheduled for palliative chemotherapy. The remaining eight patients showed LTC during the follow-up period. Figure 2 presents a representative course in a patient with successful LTC. 5 of 10 patients (50%) showed systemic tumour progression either as new lymph node metastases or as new distant metastases. The mean progression-free interval was 9.2 months (range 2–20 months).
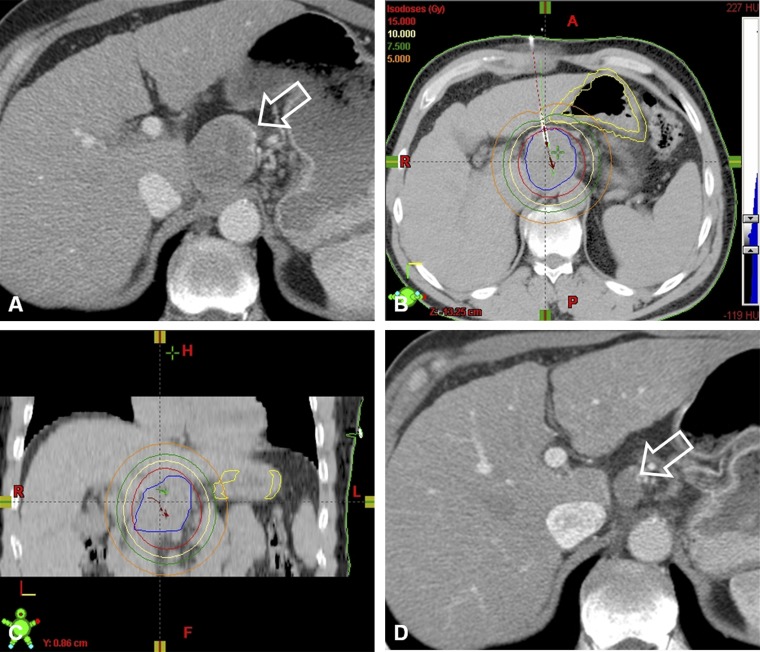
Figure 2.
Local tumour control in a 73-year-old patient with nodal metastasis of a previously treated hepatocellular carcinoma. (a) Pre-operative contrast-enhanced CT shows a bulky nodal metastasis (arrow). (b,c) The tumour was treated by CT-guided high-dose-rate brachytherapy using two catheters. (d) The follow-up contrast-enhanced CT at 12 months shows the nodal metastasis with a considerable decrease in size (arrow).
DISCUSSION
The lymph nodes are among the most common sites of extrahepatic recurrence of many abdominal tumours [1]. Bulky lymph node metastases represent a particular therapeutic challenge especially in heavily pre-treated patients and in patients with multiple comorbidities.
The role of chemotherapy in the treatment of lymph node metastases is controversial and largely dependent on the tumour type, previous treatments and other patient- and tumour-related factors. Although surgical resection of isolated recurrent lymph node metastases has proven to be effective in selected cases, the majority of patients with nodal recurrence are not amenable to surgery because of previous abdominal surgery or poor performance status [5,6]. Moreover, because nodal metastases are often difficult to access and located close to structures at high risk of damage (e.g. bowel, vessels and hilar structures), local treatment, such as surgery or radiation therapy, is often complex and associated with a high rate of complications. Zeng et al [7] reported their experience with local limited external beam radiotherapy in 62 patients with lymph node metastases from HCC. The tumour dose ranged from 40 Gy to 60 Gy in daily 2.0-Gy fractions, five times weekly. Despite encouraging results in terms of tumour response (complete response, 59.7%; partial response, 37.1%), reported complication rates appear relatively high. 13 (21%) patients developed gastroduodenal ulcers and 6 patients died of gastrointestinal bleeding.
More satisfactory results, especially in terms of reduction of complications, have been reported with the use of stereotactic body radiotherapy (SBRT). SBRT refers to an emerging radiotherapy procedure that permits accurate delivery of large doses of radiation in a few fractions. Two recent studies have shown satisfactory results in the treatment of isolated abdominal and pelvic nodal metastases by means of SBRT. Jereczek-Fossa et al [14] were able to obtain durable disease control in 20% of 64 patients treated and an infield control rate of 75.3% after a median follow-up of 20 months. Acute and late toxicity after SBRT were seen in 34.0% and 11.6% of patients, respectively. In a further recent study, Alongi et al [15] reported an overall response rate of 82% and no local progression in 25 patients with isolated abdominal lymph node metastases. None of the patients displayed severe, acute or late toxicity.
Minimally invasive tumour ablation techniques have gained great acceptance in integrated cancer treatment and have taken on an important role mainly in the management of complex patients in whom other treatments have failed or no longer represent a valid option. In recent years, several authors have reported satisfactory results for the treatment of tumours in several locations [16]. However, data regarding the use of minimally invasive ablation techniques for the treatment of nodal metastases are scarce. Mack et al [17] first reported the use of a minimally invasive tumour ablation technique for the treatment of recurrent nodal metastasis: in a series of 11 patients with recurrent extrahepatic abdominal tumours treated with laser-induced thermotherapy (LITT), 5 patients had lymph node metastases. In this initial clinical report, the authors concluded that LITT is a practicable, minimally invasive and well-tolerated technique that is able to achieve tumour inactivation and/or reduction of tumour bulk in lesions <5 cm in diameter. Following this first report, other investigators have explored the use of other thermal ablative techniques for the treatment of metastatic lymphadenopathy: Gervais et al [18] first described the use of RFA in four patients with isolated foci of nodal metastases (one retroperitoneal lymph node, two retrocrural nodes and one left obturator node), demonstrating the feasibility of minimally invasive ablation of nodal metastases with RFA. A few years later, Hiraki et al [19] described the use of RFA in treating seven metastatic mediastinal lymph nodes with concomitant cooling and temperature monitoring of the tracheal mucosa to prevent tracheal damage. After a median follow-up of 12 months, five of the seven patients had experienced local progression. Complications included two patients with pneumothorax, two with tracheal perforation and two with Horner syndrome. More encouraging results were presented in 2010 by Arellano et al [20], who reported their experience with RFA of retroperitoneal metastatic disease in eight patients with gynaecological malignancies. Three patients were excluded owing to extensive metastatic disease at the time of initial consultation (n=1) or owing to proximity of an at-risk structure (n=2). Three of the five remaining patients showed no evidence of recurrent or new disease at positron emission tomography/CT at 23.5 months after RFA. Herein, we present our first clinical experience with CT-HDRBT of 10 isolated lymph node metastases in 10 consecutive patients with recurrent nodal metastasis from different primaries. Our results show that CT-HDRBT is a feasible, safe and effective local treatment for attaining LTC in patients with abdominal nodal metastases. The most important feature that characterises CT-HDRBT is its accuracy in dose administration, which enables adequate coverage of the lesion while at the same time reducing radiation exposure of neighbouring risk structures. This feature has proven very beneficial in this group of patients and allowed us to treat even those patients with nodal recurrence in regions at high risk of complications. In fact, previous studies have demonstrated that ablation of lesions close to at-risk structures, such as bowel, bladder or hilar structures, may result in severe thermal injury, including perforation, bile leaks and strictures or tracheal/bronchial damage [19,21,22].
Furthermore, the proximity of lymph node metastases to large vessels (e.g. aorta, vena cava and iliac vessels) may prevent complete tumour ablation. In fact, it has long been known that convective cooling by adjacent blood vessels can impair the outcome of RFA by dissipating heat before complete tumour ablation is achieved [22]. This phenomenon, known as the “heat-sink effect”, limits the use of thermal ablative techniques in the vicinity of large vessels (≥3 mm in diameter) but has no impact on the effectiveness of CT-HDRBT, which does not use thermal energy [8].
The goal of the present study was to explore whether LTC can be attained using CT-HDRBT in heavily pre-treated patients with isolated lymph node metastases. The results of our retrospective analysis are convincing: after a median follow-up of 13.2 months, only 2 of the 10 treated nodal metastases showed local progression. Furthermore, none of the patients developed minor or major complications although, as already discussed above, many of the treated metastases were in locations very difficult to access and at high risk of complications owing to the vicinity of at-risk structures.
Several limitations to this pilot study need to be acknowledged. The main weaknesses of the study are the retrospective nature and the fact that our results are based on a small and heterogeneous cohort of selected patients. Accordingly, there is no reliable evidence of definitive benefit to patients, and careful patient selection and review of their suitability for alternative treatments at an interdisciplinary tumour board are mandatory. Despite significant limitations, the results of this investigation show that, owing to the accurate dose application, CT-HDRBT can be safely used for LTC in selected patients with bulky nodal metastases.
REFERENCES
- 1.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med 2008;132:931–9 10.1043/1543-2165(2008)132[931:MPOCRF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 2.Pulitanò C, Bodingbauer M, Aldrighetti L, Choti MA, Castillo F, Schulick RD, et al. Colorectal liver metastasis in the setting of lymph node metastasis: defining the benefit of surgical resection. Ann Surg Oncol 2012;19:435–42 10.1245/s10434-011-1902-1 [DOI] [PubMed] [Google Scholar]
- 3.Sawayama H, Hayashi N, Honda S, Baba Y, Toyama E, Watanabe M, et al. Treatment results of FOLFOX chemotherapy before surgery for lymph node metastasis of advanced colorectal cancer with synchronous liver metastasis: the status of LN metastasis and vessel invasions at the primary site in patients who responded to FOLFOX. Int J Clin Oncol 2010;15:70–6 [DOI] [PubMed] [Google Scholar]
- 4.Morice P, Joulie F, Rey A, Atallah D, Camatte S, Pautier P, et al. Are nodal metastases in ovarian cancer chemoresistant lesions? Analysis of nodal involvement in 105 patients treated with preoperative chemotherapy. Eur J Gynaecol Oncol 2004;25:169–74 [PubMed] [Google Scholar]
- 5.Santillan A, Karam AK, Li AJ, Giuntoli R, 2nd, Gardner GJ, Cass I, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol 2007;104:686–90 10.1016/j.ygyno.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 6.Boorjian SA, Crispen PL, Lohse CM, Leibovich BC, Blute ML. Surgical resection of isolated retroperitoneal lymph node recurrence of renal cell carcinoma following nephrectomy. J Urol 2008;180:99–103 10.1016/j.juro.2008.03.025 [DOI] [PubMed] [Google Scholar]
- 7.Zeng ZC, Tang ZY, Fan J, Qin LX, Ye SL, Zhou J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys 2005;63:1067–76 10.1016/j.ijrobp.2005.03.058 [DOI] [PubMed] [Google Scholar]
- 8.Ricke J, Wust P. Computed tomography-guided brachytherapy for liver cancer. Semin Radiat Oncol 2011;21:287–93 10.1016/j.semradonc.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 9.Collettini F, Schnapauff D, Poellinger A, Deneke T, Schott E, Berg T, et al. Hepatocellular carcinoma: computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5-7 cm) and very large (>7 cm) tumours. Eur Radiol 2012;22:1101–9 10.1007/s00330-011-2352-7 [DOI] [PubMed] [Google Scholar]
- 10.Collettini F, Golenia M, Schnapauff D, Poellinger A, Denecke T, Wust P, et al. Percutaneous computed tomography-guided high-dose-rate brachytherapy ablation of breast cancer liver metastases: initial experience with 80 lesions. J Vasc Interv Radiol 2012;23:618–26 10.1016/j.jvir.2012.01.079 [DOI] [PubMed] [Google Scholar]
- 11.Wieners G, Pech M, Rudzinska M, Lehmkuhl L, Wlodarczyk W, Miersch A, et al. CT-guided interstitial brachytherapy in the local treatment of extrahepatic, extrapulmonary secondary malignancies. Eur Radiol 2006;16:2586–93 10.1007/s00330-006-0241-2 [DOI] [PubMed] [Google Scholar]
- 12.Ricke J, Thormann M, Ludewig M, Jungnickel K, Grosser O, Wybranski, et al. MR-guided liver tumor ablation employing open high-field 1.0T MRI for image-guided brachytherapy. Eur Radiol 2010;20:1985–93 10.1007/s00330-010-1751-5 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S377–90 [DOI] [PubMed] [Google Scholar]
- 14.Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, et al. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am J Clin Oncol. doi: 10.1097/COC.0b013e3182610878. Sep 2012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Alongi F, Fogliata A, Clerici E, Navarria P, Tozzi A, Comito T, et al. Volumetric modulated arc therapy with flattening filter free beams for isolated abdominal/pelvic lymph nodes: report of dosimetric and early clinical results in oligometastatic patients. Radiat Oncol 2012;5:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillams A. Tumour ablation: current role in the liver, kidney, lung and bone. Cancer Imaging 2008;8.(Spec No. A):S1–5 10.1102/1470-7330.2008.9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack MG, Straub R, Eichler K, Roggan A, Böttger M, Woitaschek D, et al. MR-guided laser-induced thermotherapy in recurrent extrahepatic abdominal tumors. Eur Radiol 2001;11:2041–6 10.1007/s003300100966 [DOI] [PubMed] [Google Scholar]
- 18.Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of nodal metastases. Cardiovasc Intervent Radiol 2002;25:547–9 10.1007/s00270-002-2661-y [DOI] [PubMed] [Google Scholar]
- 19.Hiraki T, Yasui K, Mimura H, Gobara H, Mukai T, Hase S, et al. Radiofrequency ablation of metastatic mediastinal lymph nodes during cooling and temperature monitoring of the tracheal mucosa to prevent thermal tracheal damage: initial experience. Radiology 2005;237:1068–74 [DOI] [PubMed] [Google Scholar]
- 20.Arellano RS, Flanders VL, Lee SI, Mueller PR, Gervais DA. Imaging-guided percutaneous radiofrequency ablation of retroperitoneal metastatic disease in patients with gynecologic malignancies: clinical experience with eight patients. AJR Am J Roentgenol 2010;194:1635–8 [DOI] [PubMed] [Google Scholar]
- 21.Raman SS, Aziz D, Chang X, Ye M, Sayre J, Lassman C, et al. Minimizing central bile duct injury during radiofrequency ablation: use of intraductal chilled saline perfusion—initial observations from a study in pigs. Radiology 2004;232:154–9 [DOI] [PubMed] [Google Scholar]
- 22.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 2002;178:47–51 10.2214/ajr.178.1.1780047 [DOI] [PubMed] [Google Scholar]