Introduction
Asthma is the leading chronic illness of childhood,1,2 disproportionately affecting minority children.3 Non-Hispanic African American (14.6%) and Puerto Rican Hispanic (18.4%) children not only have higher rates of asthma prevalence than Caucasian (8.2%) children,3 but also suffer worse asthma outcomes.4 African American (22%) and Hispanic (14.1%) children visit the emergency room at a greater rate than Caucasian (9%) children.4 Age also constitutes an important risk factor for poor asthma outcomes, with adolescents ages 11–17 experiencing higher asthma death rates than younger children.2,5
Adolescents differ from younger children biologically in terms of puberty and body size, and behaviorally in terms of their independence, mobility, and risk-taking behavior.6,7 Adolescents’ preference for immediate gratification over future benefits and willingness to tolerate outcomes uncertainty contribute to significant under-adherence to daily controller medication.8 Adherence in younger children is mainly controlled by caregivers, but adolescents usually manage their own medications with only limited parental/guardian involvement.9 This situation creates distinct challenges from those in younger children and consequently necessitates different intervention strategies. The investigators used cross-sectional baseline data from a randomized controlled trial of urban adolescents to assess the influence of factors possibly associated with lower levels of adherence among African American and Hispanic adolescents ages 11–16 with persistent asthma and prescribed daily inhaled corticosteroid (ICS) medication.
Methods
Study Description and Eligibility
For this analysis, the investigators focused on data from a randomized behavioral controlled trial that assessed the efficacy of a coping peer support intervention in increasing adherence to daily ICS medication among low-income minority adolescents. Results of the trial have been published previously.10 Prior to randomization, participants completed a 3-week run-in period to assess baseline characteristics and determine eligibility for participation in a 10-week active treatment phase. Adherence to ICS was measured over the last 14 days of the 21 day run-in period using an objective electronic adherence monitor. This analysis focuses on differences in baseline characteristics between participants with low adherence to ICS at baseline versus those with high adherence (see “Measures” section below).
To be eligible for the run-in phase, participants had to be 11 to 16 years of age, diagnosed with persistent asthma, self-identified as African American or Hispanic, and in possession of an active prescription for an ICS for asthma. Persistent asthma was defined as: symptoms of asthma > 2 days/week or nighttime symptoms > 2 nights/month, or being on a daily prescription ICS for the treatment of asthma.11 Participants were excluded if the caregiver or child was unable to speak English or the child had comorbidities that would interfere with study participation. The Rush University Medical Center Institutional Review Board approved the study protocol.
Patients were recruited for the study between May 24, 2011 and February 28, 2012 from pediatric, medicine-pediatric, and family medicine practices at Rush University Medical Center in Chicago, Illinois. Eligible patients and their guardians provided written assent and consent.
Measures
After providing consent, participants entered a 3-week run in period in which eligibility and baseline adherence to ICS was assessed. Adherence was objectively measured via an electronic medication monitor (Doser CT, MediTrack, Inc., South Easton, MA) secured onto their ICS. Although they were blinded to the reason for the monitoring, participants and their guardians were instructed that the Doser CT would record the total number of actuations of their ICS each day. The Doser CT settings did not allow the participant to see the number of actuations.
For each study participant, the following formula was used to calculate their daily adherence over each 24-hour interval: adherence = (total puffs actuated/total puffs prescribed) × 100%.12 As true adherence may be distorted by intentional overuse (such as by medication dumping), this daily adherence measure was truncated at 100%.13,14 To allow adoption of a standard electronic dose counter adherence measure across all study participants, the investigators switched participants on different ICS medications at enrollment to a best estimation dose of Flovent HFA 110 mcg Inhalation Aerosol (provided by GlaxoSmithKline, Research Triangle Park, NC). All participants were provided with spacers, and taught proper ICS medication inhalation technique.15 Baseline adherence was defined as the average daily adherence over the last 14 days prior to completion of the 3-week run-in period. For this analysis, a participant was designated as having low adherence if their baseline adherence (i.e. average daily adherence at baseline) was ≤ 48%. This designation was based on the fact that most participants (82%) were prescribed 2 puffs of ICS medication twice daily, or 56 puffs over a 14 day period. The investigators considered taking >48% of prescribed puffs over a 14 day period as representing high adherence (e.g. at least 27 of 56 puffs) and taking ≤ 48% of prescribed puffs (e.g. less than or equal to 26 of 56 puffs) as representing low adherence. Observational studies that electronically monitored adherence to daily ICS in diverse samples of adolescents report rates between 40–50% as being typical.16–19
Participant assessments included: demographic, asthma history, asthma control,11 asthma exacerbation,11 asthma knowledge,20 ICS knowledge,13 depression,21 and ICS self-efficacy.22
Asthma Variables
Possession of an active prescription for ICS medication was verified by having the participant bring in the medication with his or her name on the pharmacy label, or having the pharmacy confirm availability of an active prescription. Asthma control was ascertained using questions from the domains of impairment and risk from the NHLBI EPR3 guidelines as follows: recall of prior 2–4 weeks of daytime symptoms, nighttime awakenings, interference with normal activities, use of short acting beta-2 agonists for symptom control, and number of asthma exacerbations warranting use of oral systemic steroids in the past 12 months.11 Self-reported tobacco smoke exposure was queried. The ZAP Asthma Knowledge Instrument, a 39-item questionnaire adapted from the ZAP Caregiver Asthma Knowledge Survey Instrument, was used to measure asthma knowledge in the adolescents.21 ICS knowledge for the adolescents was assessed using the 7-item Inhaled Corticosteroid Knowledge Questionnaire which measures knowledge of the function of ICS.13 Asthma exacerbations in the past 12-months were defined as self-reported number of: days absent from school; oral corticosteroid bursts; unscheduled urgent outpatient visits to the doctor; emergency department visits; hospitalizations; admissions to the intensive care unit; and intubations.11
Psychosocial Variables
Levels of depression were evaluated using the Children’s Depression Inventory 2.20 The 12-item Inhaled Corticosteroid Self-Efficacy Questionnaire was used to measure participant perceived self-efficacy at taking ICS.22
Statistical Methods
Participants determined to have low adherence at baseline were compared to those determined to have high adherence with respect to key covariates. Continuous variables were compared using the Wilcoxon rank sum test; discrete variables were compared using the Fisher Exact test. Univariate logistic regression was used with respect to each covariate identified above to identify variables significantly associated with low adherence. Those variables found to be significant with p < 0.20 were then included in a forward stepwise selection logistic regression analysis to identify the subset of variables most significantly associated with the low adherence categorization. The stepwise analysis employed a criterion of p ≤ 0.10 for a variable to be entered into the model. Adequacy of the resulting final model was assessed using the Hosmer-Lemeshow goodness-of-fit test. All statistical analyses were performed using SAS v.9.2 software.
Results
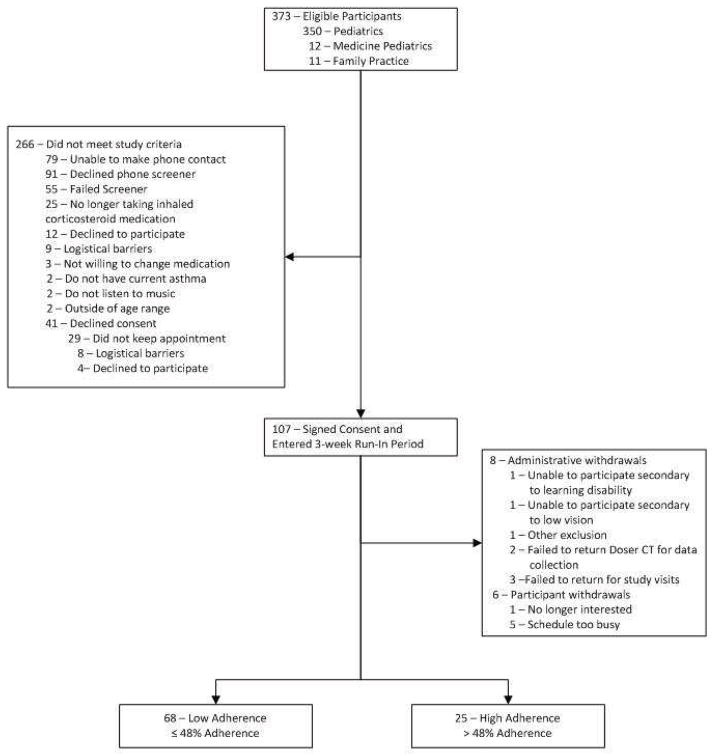
Details of the flow of participants from eligibility to completion of the three week run-in period are presented in Figure 1. A search of the electronic medical record identified a total of 373 potentially eligible patients. Of these, 266 did not meet study inclusion criteria and 107 completed informed assent and consent procedures. Fourteen participants did not complete the run-in, eight due to administrative withdrawal and six due to participant withdrawal, resulting in 93 subjects with data available for this analysis.
Figure 1.
CONSORT diagram illustrating participant flow from eligibility ascertainment through completion of objective adherence assessment
Table 1 summarizes potential demographic, asthma, psychosocial and media use factors associated with adherence for the 93 participants included in this analysis, by adherence category (low vs. high). Those with low adherence differed from those with high adherence on three characteristics. Those with low adherence were older (mean age = 13.4 years vs. 12.4 years, p = 0.012) and disproportionately male (51.5% in low adherence group vs. 24% in high adherence group, p = 0.020). Those designated with high adherence had significantly higher ICS knowledge (p <0.001) than those with low adherence.
Table 1.
Comparison of Potential Factors Between Low Adherence versus High Adherence Groups
| Total (n=93) | Low Adherence (≤48%) (n=68) | High Adherence (>48%) (n=25) | p value | |
|---|---|---|---|---|
| DEMOGRAPHIC | ||||
| Age Mean (Min, Max) | 13.2 (10, 16) | 13.4 (11,16) | 12.4 (10, 16) | 0.012 |
| Gender, n (%) | ||||
| Male | 41 (44.1) | 35 (51.5) | 6 (24.0) | 0.020 |
| Female | 52 (55.9) | 33 (48.5) | 19 (76.0) | |
| Ethnicity: Hispanic/Latino, n (%) | 10 (10.8) | 8 (11.8) | 2 (8.0) | 0.724 |
| Race, n (%) | ||||
| Black/African American | 82 (88.1) | 59 (86.8) | 23 (92.0) | 0.797 |
| Mixed African American* | 1 (1.1) | 1 (1.5) | 0 (0.0) | |
| Other | 10 (10.8) | 8 (11.8) | 2 (8.0) | |
| Child insurance status, n (%) | ||||
| Public | 70 (75.3) | 53 (77.9) | 17 (68.0) | 0.417 |
| Private | 23 (24.7) | 15 (22.1) | 8 (32.0) | |
| Receive free or reduced school lunch, † n (%) | 76 (84.4) | 55 (82.1) | 21 (91.3) | 0.505 |
| ASTHMA | ||||
| Asthma controller medications, n (%) | ||||
| ICS monotherapy | 71 (76.3) | 51 (75.0) | 20 (80.0) | 0.785 |
| ICS and long-acting bronchodilator combination therapy‡ | 22 (23.7) | 17 (25.0) | 5 (20.0) | |
| Smoking behavior reported by adolescent, n (%) | ||||
| Current smoker | 2 (2.1) | 2 (2.9) | 0 (0.0) | >0.999 |
| Exposed to second hand smoke at home | 6 (6.5) | 5 (7.4) | 1 (4.0) | >0.999 |
| ZAP Asthma Knowledge Instrument | ||||
| % items correct, median (Q1, Q3) | 69.2 (61.5, 74.4) | 69.2 (61.6, 76.9) | 66.7 (61.5, 71.8) | 0.260 |
| ICS Knowledge Questionnaire, median (Q1, Q3) | 25.0 (23.0, 27.0) | 24.0 (22.0, 26.0) | 26.0 (24.0, 29.0) | <0.001 |
| PSYCHOSOCIAL | ||||
| Children’s Depression Inventory 2 n, (%) | ||||
| Very elevated | 15 (16.1) | 11 (16.2) | 4 (16.0) | 0.976 |
| Elevated | 6 (6.5) | 4 (5.9) | 2 (8.0) | |
| High average | 11 (11.8) | 8 (11.8) | 3 (12.0) | |
| Average or lower | 61 (65.6) | 45 (66.2) | 16 (64.0) | |
| ICS Self-Efficacy Questionnaire, median (Q1, Q3) | 53.0 (47.0, 59.0) | 51.5 (46.5, 59.0) | 55.0 (49.0, 60.0) | 0.245 |
Mixed African American: Self-identified as a combination of African American and another race
Criteria for Free and Reduced School Lunch in Chicago Public Schools July 1, 2011 - June 30, 2012. Source: http://www.fns.usda.gov/cnd/governance/notices/iegs/IEGs11-12.pdf
Inhaled corticosteroid and long-acting bronchodilator: Two participants who were taking Inhaled Corticosteroid and Long Acting Bronchodilator Combination Therapy were also taking Inhaled Corticosteroid Monotherapy
Table 2 presents asthma control and morbidity information. Despite all being prescribed daily ICS by their primary care physician, 84% of the participants had uncontrolled asthma. Thirty-two percent of those with low adherence vs. 40% of those with high adherence reported ≥ 2 asthma exacerbations requiring oral systemic corticosteroids within the last 12 months (p= 0.623). Those with high adherence were somewhat more likely than those with low adherence to have reported ≥ 1 asthma-related emergency room visit or hospitalization in the previous 12 months (75% vs. 52%, p = 0.056).
Table 2.
Asthma Control and Morbidity
| Total (n=93) | Low Adherence (≤48%) (n=68) | High Adherence (>48%) (n=25) | p value | |
|---|---|---|---|---|
| Uncontrolled Asthma, n (%)* | 78 (83.9) | 56 (82.4) | 22 (88.0) | 0.752 |
| Asthma exacerbation in the last 12 months | ||||
| ≥ 2 Requiring oral systemic corticosteroids, n (%) | 32 (34.4) | 22 (32.4) | 10 (40.0) | 0.623 |
| ≥ 1 Requiring emergency room visit or hospitalization, n (%) | 53 (57.6) | 35 (51.5) | 18 (75.0) | 0.056 |
Asthma control was assessed via participant self-report to questions developed based on the NHLBI EPR-3 Guideline impairment and risk domains.11
Table 3 presents the final multivariate model results. Univariate logistic regression analyses yielded the following variables as being significantly associated (i.e., p < 0.20) with low adherence: age, gender, race, Hispanic ethnicity (yes/no), insurance status (private vs. public), ≥ 2 exacerbations requiring oral systemic corticosteroids in the past 12 months (yes/no), received free or reduced school lunch (yes/no), ICS knowledge score, and ICS self-efficacy score. Forward stepwise selection analysis resulted in only the variables age (p = 0.004) and ICS knowledge (p = 0.01) being entered into the model. The Hosmer-Lemeshow goodness-of-fit test resulted in a p-value of 0.98, indicating that the model is quite reasonable, possessing adequate fit. This model implies that older adolescents (OR 1.739, 95% CI 1.197–2.525) with less knowledge of ICS (OR 0.813, 95% CI 0.694–0.951) were more likely to have low adherence at baseline.
Table 3.
Final Multivariate Model for Predictors of Low Adherence
| Factor | Odds Ratio | (95% CI) |
|---|---|---|
| Age | 1.739 | (1.197 – 2.525) |
| ICS Knowledge | 0.813 | (0.694 – 0.951) |
Discussion
This analysis of factors potentially associated with low adherence to daily ICS medication within a sample of minority adolescents with persistent asthma identified older age and low knowledge of ICS as being significant, after adjusting for other baseline characteristics. The inverse relationship between age and adherence may appear counterintuitive. At face value, one would think that older age would lead to high adherence to daily ICS medication. The findings of this study are consistent with those of a study by McQuaid and colleagues17 that, in a diverse sample of adolescents ages 8–16 prescribed daily ICS, although older adolescents assumed increased responsibility for medication taking behavior, objectively measured adherence declined with age.17 In a study investigating the age at which a diverse sample of children started taking responsibility for medication taking behavior, by age 11 children had, on average, assumed 50% of daily asthma controller medication responsibility.9 As adolescents increase in age, asthma medication taking responsibility transfers from the parent/guardian to the child.9 At the same time, adolescents’ complacency with outcomes uncertainty and drive for instant gratification over delayed benefits may contribute to nonadherence to daily ICS.8
Increasing asthma knowledge and knowledge of ICS are national asthma treatment guideline goals; 11 a primary or key secondary outcome of asthma studies in urban minority children and adolescents; 23, 24 and an important element of the clinician-patient encounter. The association of poor knowledge of ICS with low adherence is not surprising, and suggests sustained and increased efforts to educate adolescent asthma patients about ICS are needed.
This study has several important limitations. Minority status, 13,14,17,25,26 parental attitudes, 27 and family dysfunction14, 28 have also been associated with low adherence, but were not examined in this study. All participants belonged to a minority group as part of the study inclusion criteria, and thus there is no comparison of adherence between minority and nonminority adolescents. As the study included predominantly African American compared to Hispanic adolescents, the findings may not be generalizable to a Hispanic population. Parental attitudes and family dysfunction were not directly measured, as this study focused on the adolescent only and did not intervene at the family level. This was done purposefully as adolescence is the key developmental stage when lifelong health behaviors and coping skills are developed, 29–31 and more interventions directly targeting the adolescent are needed. However, the investigators may now rethink their approach, and while continuing to focus on the adolescent, also seek parent/guardian involvement in future studies.
Neither poverty13 nor depression, 32–34 both of which have been associated with low adherence to asthma controller medications in adolescents in prior studies, demonstrated a significant association with low adherence in this study. Public insurance status and receipt of free or reduced school lunch were used as indicators of poverty. In the high adherence group, 68% had public insurance, and 91% received free or reduced school lunch. In the low adherence group, 78% had public insurance, and 82% received free or reduced school lunch. While our study was successful in targeting recruitment of disadvantaged minority adolescents, the lack of income diversity in our sample may have precluded us from discerning an association between poverty and adherence.
In contrast to prior research, this study only measured depression at one point in time, and only measured it in the adolescent, rather than child and caregiver. Future studies would benefit from gathering data on the child’s mood at the time of depression instrument completion, as well as at the time of medication taking behavior (or lack there-of) through ecological momentary assessment, and also assessing caregiver depression. Individuals may be more likely to report symptoms of depression, and not adhere to medication, at times of distressed emotional state.18 Although not assessed in this study, caregiver depression has been associated with poor adherence to ICS among low income minority adolescents with asthma.35 The relationship between adolescent, parental, as well as adolescent and parental depression and adherence to ICS merits further investigation.
A major strength of this study is the objective measurement of adherence data. Pediatric studies using both self-report and objective measures to capture adherence to ICS demonstrate significant overestimation of adherence by the former approach.10,36,37 It is crucial to have accurate adherence data in order to draw reliable conclusions between levels of adherence and potentially associated factors.
In conclusion, this study makes important research and clinical contributions by demonstrating an association between low objectively measured adherence to ICS with older age and poor knowledge of ICS in urban minority adolescents. It is important for researchers and clinicians not to assume that increased responsibility for medication taking behavior in adolescents translates into greater adherence to ICS.17 Our findings promote efforts to sustain and expand asthma education in minority adolescents to increase their controller medication taking behavior.11,24,25
Acknowledgments
Funding: This study was supported by the National Heart Lung and Blood Institute grants K23 HL092292 and R21 HL098812. Support in the form of study drug was provided by a grant from GlaxoSmithKline (FLV114794).
Abbreviations
- ICS
Inhaled corticosteroids
- ATG
Attention control group
- mp3
Music file (MPEG Layer 3)
Footnotes
Author Contributions: Doctors Mosnaim, Li, Martin, Richardson, Ryan, Bender, and Powell; as well as Ms. Belice and Ms. Avery all contributed toward conception and design of the study, data generation, data analysis and interpretation, and manuscript preparation.
www.clinicaltrials.gov Clinical Trials.gov Identifier: NCT01169883
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Vital Health Stat 10. 1999;200:1–211. [PubMed] [Google Scholar]
- 2.Akinbami LJ. The state of childhood asthma, United States, 1980–2005. National Center for Health Statistics Vital Health Stat. 2006;381:1–22. [Google Scholar]
- 3.Moorman JE, Zahran H, Truman B, Molla M. Current Asthma Prevalence – United States, 2006–2008. Centers for Disease Control & Prevention MMWR. 2011;60(Suppl):84–6. [PubMed] [Google Scholar]
- 4.Moorman JE, Rudd RA, Johnson CA, et al. National Surveillance for Asthma – United States, 1980 – 2004. Centers for Disease Control & Prevention. MMWR. 2007;56(SS-8) [PubMed] [Google Scholar]
- 5.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: Prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–22. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 6.Adams PF, Schoenborn CA, Moss AJ, Warren CW, Kann L. Health-risk behaviors among our nations’ youth: United States, 1992. Vital Health Stat 10. 1995;192:1–51. doi: 10.1037/e609452007-001. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance-United States, 2011. MMWR. 2012;61(SS-4):1–162. [PubMed] [Google Scholar]
- 8.Brandt S, Dickinson B. Time and risk preferences and the use of asthma controller medication. Pediatrics. 2013;131:e1204–e1210. doi: 10.1542/peds.2011-2982. [DOI] [PubMed] [Google Scholar]
- 9.Orrell-Valente JK, Jarslberg LG, Hill LG, Cabana MD. At what age do children start taking daily asthma medicines on their own? Pediatrics. 2008;122:e1186–e1192. doi: 10.1542/peds.2008-0292. [DOI] [PubMed] [Google Scholar]
- 10.Mosnaim G, Li H, Martin M, et al. The impact of peer support and mp3 messaging on adherence to inhaled corticosteroids in minority adolescents with asthma: A randomized, controlled trial. J Allergy Clin Immunol: In Practice. 2013;1:485–93. doi: 10.1016/j.jaip.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethedsa, MD: National Institutes of Health; 2007. pp. 1–440. Full Report. [Google Scholar]
- 12.Krishnan JA, Riekert KA, McCoy JV, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170:1281–5. doi: 10.1164/rccm.200403-409OC. Epub 2004 Sep 16. [DOI] [PubMed] [Google Scholar]
- 13.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids: Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157:1810–7. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 14.Bender B, Wamboldt FS, OConnor SL, et al. Measurement of children’s asthma medication adherence by self-report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85:416–21. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 15.American College of Chest Physicians Patient Education Guide: using your MDI with a spacer [Internet] American College of Chest Physicians; 2006. Available from: http://www.chestnet.org/downloads/patients/guides/inhaledDevices/patientEducation8.pdf. [Google Scholar]
- 16.Naimi D, Freedman T, Ginsburg K, Bogen D, Rand C, Apter A. Adolescents and asthma: Why bother with our meds? J Allergy Clin Immunol. 2009;123:1335–41. doi: 10.1016/j.jaci.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28:323–33. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 18.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. Journal of Allergy and Clinical Immunol. 2008;122:490–5. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 19.Walders N, Kopel S, Koinis-Mitchell D, McQuaid EL. Patterns of quick-relief and long-term controller medication use in pediatric asthma. J Pediatrics. 2005;146:177–82. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–15. [PubMed] [Google Scholar]
- 21.ZAP Asthma Project. Caregiver asthma knowledge survey instrument, Form 9 [Internet] Atlanta, GA: [revised 1997 Oct 3; cited 2007 Jun 8]. Available from: http://www.sph.emory.edu/zapasthma/pdf/a9.pdf. [Google Scholar]
- 22.Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: It’s not just black and white. J Allergy Clin Immunol. 2003;111:1219–26. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 23.Mosnaim GS, Li H, Damitz M, et al. Evaluation of the Fight Asthma Now (FAN) program to improve asthma knowledge in urban youth and teenagers. Ann Allergy Asthma Immunol. 2011;107:310–6. doi: 10.1016/j.anai.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Mosnaim GS, Cohen MS, Rhoads CH, Rittner SS, Powell LH. Use of mp3 players to increase asthma knowledge in inner-city African-American adolescents. Int J Behav Med. 2008;15:341–46. doi: 10.1080/10705500802365656. [DOI] [PubMed] [Google Scholar]
- 25.Cooper WO, Hickson GB. Corticosteroid prescription filling for children covered by Medicaid following an emergency department visit or a hospitalization for asthma. Arch Pediatr Adolesc Med. 2001;155:1111–15. doi: 10.1001/archpedi.155.10.1111. [DOI] [PubMed] [Google Scholar]
- 26.Burgess S, Sly P, Devadason S. Adherence with preventive medicine in childhood asthma. Pulm Med. 2011;2011:1–6. doi: 10.1155/2011/973849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan PW, DeBruyne JA. Parental concern towards the use of inhaled therapy in children with chronic asthma. Pediatr Int. 2000;42:547–51. doi: 10.1046/j.1442-200x.2000.01278.x. [DOI] [PubMed] [Google Scholar]
- 28.Bender B, Milgrom H, Rand C, Ackerson L. Psychological factors associated with medication nonadherence in asthmatic children. J Asthma. 1998;35:347–353. doi: 10.3109/02770909809075667. [DOI] [PubMed] [Google Scholar]
- 29.van Es SM, Nagelkerke AF, Colland VT, Scholten RJ, Bouter LM. An intervention programme using the ASE-model aimed at enhancing adherence in adolescents with asthma. Patient Educ Couns. 2001;44:193–203. doi: 10.1016/s0738-3991(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 30.Williams PG, Holmbeck GN, Greenley RN. Adolescent health psychology. J Consult Clin Psychol. 2002;70:828–42. [PubMed] [Google Scholar]
- 31.Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: The adolescent experience. Patient Educ Couns. 2004;55:396–406. doi: 10.1016/j.pec.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Smith A, Krishnan J, Bilderback A, Riekert K, Rand C, Bartlett S. Depressive symptoms and adherence to asthma therapy after hospital discharge. Chest. 2006;130:1034–8. doi: 10.1378/chest.130.4.1034. [DOI] [PubMed] [Google Scholar]
- 33.Cluley S, Cochrane G. Psychological disorder in asthma is associated with poor control and poor adherence to inhaled steroids. Respir Med. 2001;95:37–39. doi: 10.1053/rmed.2000.0968. [DOI] [PubMed] [Google Scholar]
- 34.Baiardini I, Braido F, Giardini A, et al. Adherence to treatment: Assessment of an unmet need in asthma. J Investig Allergol Clin Immunol. 2006;16:315–25. [PubMed] [Google Scholar]
- 35.Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics. 2004;113:229–237. doi: 10.1542/peds.113.2.229. [DOI] [PubMed] [Google Scholar]
- 36.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98:1051–7. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan J, Bender B, Wamboldt F, et al. Adherence to inhaled corticosteroids: An ancillary study of the Childhood Asthma Management Program clinical trial. J Allergy Clin Immunol. 2012;129:112–8. doi: 10.1016/j.jaci.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]