Table 1.
Different activators in the rearrangement of 1 a[a]
 | |||
|---|---|---|---|
| Entry | Activator | 2 aYield [%] | 3 aYield [%] |
| 1 | – | 0 | 0 |
| 2 | TMSOTf | 93 | 0 |
| 3 | TBDMSOTf | 88 | 0 |
| 4 | BF3⋅OEt2 | 18 | 67 |
| 5[b] | camphorsulfonic acid | 0 | 92 |
| 6[c] | TsOH⋅H2O | 8 | 64 |
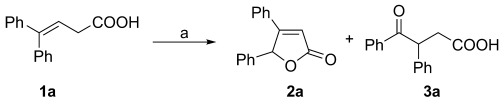
Reagents and conditions: a) PhI(OCOCF3)2 (1 equiv), activator (2 equiv), MeCN, RT, 30 min;
When the reaction was performed by using substrate (E)-1 f, rearranged product 3 f was obtained in 89 % yield.
Koser’s reagent Ph(OH)OTs was used for this reaction. In addition to 2 a and 3 a, compound 4 a was observed in 8 % yield.
