Table 2.
Oxidative rearrangements of compounds 1 a–h[a]
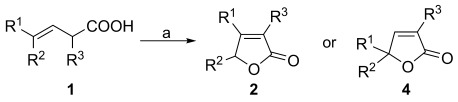 | |||
|---|---|---|---|
| Entry | Substrate1 | Yield of2[%] | Yield of4[%] |
| 1 | 1 a: R1=R2=Ph, R3=H | 93 | – |
| 2 | 1 b: R1=R2=4-Cl-C6H4, R3=H | 95 | – |
| 3 | 1 c: R1=Ph, R2=4-Br-C6H4, R3=H | 88 | – |
| 4[b] | 1 d: R1=R2=Ph, R3=Me | 81 | – |
| 5 | 1 e: R1=Ph, R2=R3=H | 78 | – |
| 6 | (E)-1 f: R1=Ph, R2=Me, R3=H | 89 | – |
| 7 | (Z)-1 f: R1=Me, R2=Ph, R3=H | trace | 81 |
| 8 | 1 g: R1=Ph, R2=R3=Me | 87 | – |
| 9[c] | 1 h: R1=Me, R2=R3=H | – | 73 |
Reagents and conditions: a) PhI(OCOCF3)2 (1 equiv), TMSOTf (2 equiv), MeCN, RT, 30 min;
The use of PhI(OH)OTs instead of PhI(OCOCF3)2 yielded 2 d in 76 % yield together with 4 d in 16 % yield.
Reaction time was 5 h.
