Abstract
The purpose of this study was to investigate blood flow and its heterogeneity within and among the knee muscles in five young (26 ± 6 years) and five old (77 ± 6 years) healthy men with similar levels of physical activity while they performed two types of submaximal fatiguing isometric contraction that required either force or position control. Positron emission tomography (PET) and [15O]-H2O were used to determine blood flow at 2 min (beginning) and 12 min (end) after the start of the tasks. Young and old men had similar maximal forces and endurance times for the fatiguing tasks. Although muscle volumes were lower in the older subjects, total muscle blood flow was similar in both groups (young men: 25.8 ± 12.6 ml min−1; old men: 25.1 ± 15.4 ml min−1; age main effect, P = 0.77) as blood flow per unit mass of muscle in the exercising knee extensors was greater in the older (12.5 ± 6.2 ml min−1 (100 g)−1) than the younger (8.6 ± 3.6 ml min−1 (100 g)−1) men (age main effect, P = 0.001). Further, blood flow heterogeneity in the exercising knee extensors was significantly lower in the older (56 ± 27%) than the younger (67 ± 34%) men. Together, these data show that although skeletal muscles are smaller in older subjects, based on the intact neural drive to the muscle and the greater, less heterogeneous blood flow per gram of muscle, old fit muscle achieves adequate exercise hyperaemia.
Key points
The results of previous studies that attempted to demonstrate the effects of ageing on skeletal muscle blood flow are controversial because these studies used indirect assessments of skeletal muscle blood flow obtained via whole limb blood flow measurements that provide no information on the distribution of blood flow within particular muscles.
We used positron emission tomography to measure blood flow per gram of muscle in old and young men with similar levels of physical activity.
Resting muscle blood flow was similar in both groups and exercising muscle blood flow was greater and less heterogeneous in the older men.
Old and young men achieved similar maximal voluntary contraction forces and endurance times during two types of fatiguing isometric task.
These findings indicate that physically active old men have intact neural drive to the muscle and achieve adequate exercise hyperaemia despite the age-induced decrease in their muscle volume.
Introduction
The magnitude or rate of decline in a performance element relative to a reference value over a given time of task performance, also known as fatigability (Kluger et al. 2013), is often reduced in old adults compared with their younger counterparts (Ditor & Hicks 2000; Hunter et al. 2004; Bilodeau et al. 2011), but this is not a universal finding (Allman & Rice 2001; Stackhouse et al. 2001). Because the mechanisms underlying fatigability depend on the specific demands of the task being performed, it cannot be generalized that muscles of old adults are more or less fatigable than those of young adults (Enoka et al. 2011).
As suggested previously (Allman & Rice, 2002; Kent-Braun, 2009), one important mechanism related to skeletal muscle fatigue in elderly subjects is muscle energetics. Accordingly, muscle blood flow is likely to play a significant role in the development of muscle fatigue because an adequate blood supply is important to the delivery of oxygen and nutrients, and the removal of fatigue-inducing metabolic byproducts from the exercising muscles. It has been suggested that an inadequate blood supply to working muscles will ultimately affect their contractile properties, resulting in a decrease in force production (Bigland-Ritchie et al. 1995). Moreover, whereas some studies have suggested that blood flow to exercising muscles decreases with age (Degens, 1998; Proctor et al. 1998), others have demonstrated no differences between age groups in blood flow to exercising muscles during unilateral or bilateral knee extension exercises (Jasperse et al. 1994; Magnusson et al. 1994; Proctor et al. 2003). Discrepancies in the literature may reflect differences in study design, including in the types of task performed and blood flow measurement techniques. For example, indirect assessment of skeletal muscle perfusion via limb blood flow measurements is likely to over- or underestimate muscle blood flow because of differences in the proportions of muscle mass, which is often decreased in older subjects, and the inability to distinguish blood flow in different tissues of the limbs (Cooper et al. 1955; Raitakari et al. 1996). Furthermore, limb blood flow measurements provide no information on the distribution of blood flow within particular muscles.
Blood flow distribution between and within working muscles may help to explain muscle fatigability and differences in fatigability between young and old adults. Although previous studies have shown that muscle blood flow is not uniformly distributed among and within working muscles in trained and untrained younger adults both at rest and during intermittent static exercise (Kalliokoski et al. 2000, 2003a, b2003b), no study has examined muscle blood flow distribution during isometric contractions in older adults. Because ageing is associated with smaller muscle size and changes in muscle contractile properties and associated vascular properties (Doherty, 2003; Kragstrup et al. 2011), blood flow distribution may change with ageing.
Positron emission tomography (PET) and [15O]-H2O provide a unique tool for the direct measurement of muscle blood flow (perfusion) in specific muscle regions, which has been used in studies concerning vascular responsiveness to sustained and intermittent knee extension (Kalliokoski et al. 2006). PET is based on the use of short-lived positron-emitting radioisotopes such as oxygen-15 (half-life: 123 s). Tracers such as [15O]-H2O are labelled with radioisotopes and applied to conduct muscle blood flow measurements. Specifically, the tracer is injected into the blood circulation and its concentration in the tissue is detected with the PET scanner. Based on the kinetics of [15O]-H2O in the arterial blood and tissues of interest, it is possible to obtain quantitative blood flow measurements in specific regions of interest (RoIs) (Iida et al. 1986; Ruotsalainen et al. 1997).
The purpose of this study was to investigate the effects of two types of fatiguing isometric contraction on blood flow and blood flow heterogeneity within and among knee extensors and flexors in healthy and equally physically active young and old men using PET and [15O]- H2O.
Methods
Subjects
Five young (26 ± 6 years) and five old (77 ± 6 years) healthy men with similar body mass (77.3 ± 5.9 kg and 79.0 ± 6.2 kg, respectively; P = 0.7) volunteered to participate in the study. Written informed consent was obtained from all subjects. Young and old men reported participating in moderate levels of structured physical activity (2–4 days per week) and engaging in activities such as endurance and strength training for approximately 1 h every second day. Furthermore, the older men reported having been physically active for their entire lives. All participants were non-smokers and reported the absence of cardiovascular and neurological disorders, and were not taking any medications. The subjects were requested to abstain from caffeine-containing beverages for at least 24 h before the experiments and to avoid strenuous exercise within 48 h prior to the experiments. The experiments were performed at least 3 h after the subjects had eaten a light breakfast. The study was performed according to the Declaration of Helsinki and was approved by the Ethical Committee of the Hospital District of South-Western Finland.
Study design
Participants visited the laboratory on four occasions spaced 1 week apart. At the first visit, subjects performed the position task with the target force set at 25% of maximal voluntary contraction (MVC) for practice and to ensure that they were able to sustain both tasks for long enough to allow the measurement of blood flow twice in the PET scanner. The position task was used because previous studies have shown it to be more difficult to sustain than the force task (Hunter et al. 2002; Rudroff et al. 2010, 2011). During the two subsequent sessions, two catheters were inserted into, respectively, an antecubital vein for tracer administration and the opposite radial artery for blood sampling. Then, subjects lay down in the PET scanner, with their femoral regions in the gantry, and performed two fatiguing contractions in a randomized order within the PET scanner. Skeletal muscle blood flow in the femoral region was measured after 2 min (beginning) and 12 min (end) using PET and [15O]-H2O (Fig. 1). The two tasks required each subject to sustain an isometric contraction with the knee extensors at 25% of MVC force for 90% of the endurance time established during the first session. One fatiguing contraction (force task) required the knee extensors to pull against a rigid restraint and to match the force exerted by the leg to the target force displayed on a monitor (1% MVC/cm). The other fatiguing contraction (position task) required the subject to use the knee extensors to support an equivalent inertial load and to maintain the position of the leg by matching knee angle to the target displayed on the monitor (1°/cm).
Figure 1.
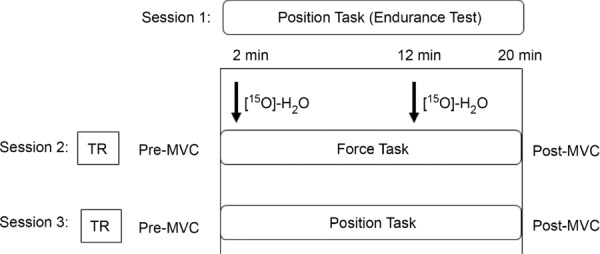
Study protocol Session 1: subjects performed an endurance test (position task). Sessions 2 and 3: after a transmission scan (TR), subjects performed a force and position task at 25% of maximal voluntary contraction (MVC) for 90% of the endurance time (∼22 min) established during the first session; muscle perfusion was measured at 2 min and 12 min using radiowater ([15O] H2O) (black arrows). During the exercises muscle force production and electromyographic activity were recorded. MVCs were measured before and immediately after each task.
The fatiguing contractions were performed with the subject in a supine position, with the trunk–thigh angle at 3.14 rad, the left knee joint angle at 0.78 rad and the right knee angle at 1.57 rad. One strap was placed around the waist to stabilize the subject. Another strap was wrapped around the ankle to connect the load to the leg. The force exerted by the leg was measured with a load cell (0–500 lb; Noraxon USA, Inc., Scottsdale, AZ, USA) placed in series with the load. The force signal was low-pass filtered (0–5 Hz) and recorded on a computer (1000 samples/s). Knee joint angle during the position task was measured with a flexible two-dimensional goniometer sensor (Noraxon USA, Inc.) secured to the lateral aspect of the knee joint. The output of the goniometer was recorded, displayed on a monitor and stored (1000 samples/s) on a computer. The inertial load (25% of MVC force) for the position task was suspended from the ankle at the location at which the restraint had been applied during the force task.
Before and immediately after each fatiguing contraction, subjects performed an MVC with the knee extensor muscles. The initial MVC was used to determine the target force (25% of MVC) for the fatiguing contraction; the final MVC was used to derive an index of fatigability. The MVC task comprised a 3 s increase in force from zero to maximum with the maximal force held for ∼3 s; subjects were verbally encouraged to achieve maximal force. Subjects rested for 60–90 s between trials. When the peak forces achieved in two of the three trials differed by >5%, additional MVCs were performed until this criterion was met. The greatest force achieved by each subject was taken as the MVC force.
Within 1 week after the PET study, structural magnetic resonance imaging (MRI) of the thigh was performed to obtain an anatomic reference of the RoIs within the PET images and to calculate quadriceps femoris muscle volumes.
Blood flow measurements and analysis
Radiowater positron-emitting tracer [15O]-H2O was produced as previously described in detail (Sipilä et al. 2001) and the ECAT EXACT HR+ scanner (Siemens Molecular Imaging, Inc., Knoxville, TN, USA) was used in three-dimensional mode for image acquisition to measure blood flow. Photon attenuation was corrected by a 5 min transmission scan performed before the beginning of both the force and position tasks (Fig. 1). All data were corrected for dead time, decay and measured photon attenuation. Images were reconstructed into a 256 × 256 matrix, producing 2.57 × 2.57 mm in-plane dimensions of voxels with 2.43 mm plane thickness. On average 1.2 ± 0.2 GBq (33 ± 4 mCi) [15O]-H2O was given 2 min and 12 min after the beginning of the task. Input function was obtained from arterial blood, which was continuously withdrawn at a constant speed with a pump. The radioactivity concentration of blood was measured using a two-channel online detector system (Scanditronix Wellhofer AB, Uppsala, Sweden) that was cross-calibrated with an automatic gamma counter (Wizard 1480; Wallac Oy, Turku, Finland) and the PET scanner.
On the PET images, RoIs were identified using cylinders with reference to the MRI images (Fig. 2). Eight RoIs were identified in the knee extensors (vastus lateralis, vastus intermedius, vastus medialis, rectus femoris) and knee flexors (biceps femoris short and long heads, semimembranosus, semitendinosus) in the thigh section, defined as 50% of the distance from the femoral head to the knee joint. PET and MRI images were then coregistered using a semi-automatic procedure. Firstly, VINCI software (Max Planck Institute for Neurologic Research, Cologne, Germany) was used to align the images along the z-axis and to obtain a valid approximation of the final coregistration. Secondly, manual tuning using bones and blood vessels as references was performed. The data were analysed with the software packages Carimas (Cardiac Image Analysis System), developed at the Turku PET Centre (Nesterov et al. 2009), and Analyze Version 11.0 (Biomedical Imaging Resource Group, Mayo Clinic, Rochester, MN, USA).
Figure 2.
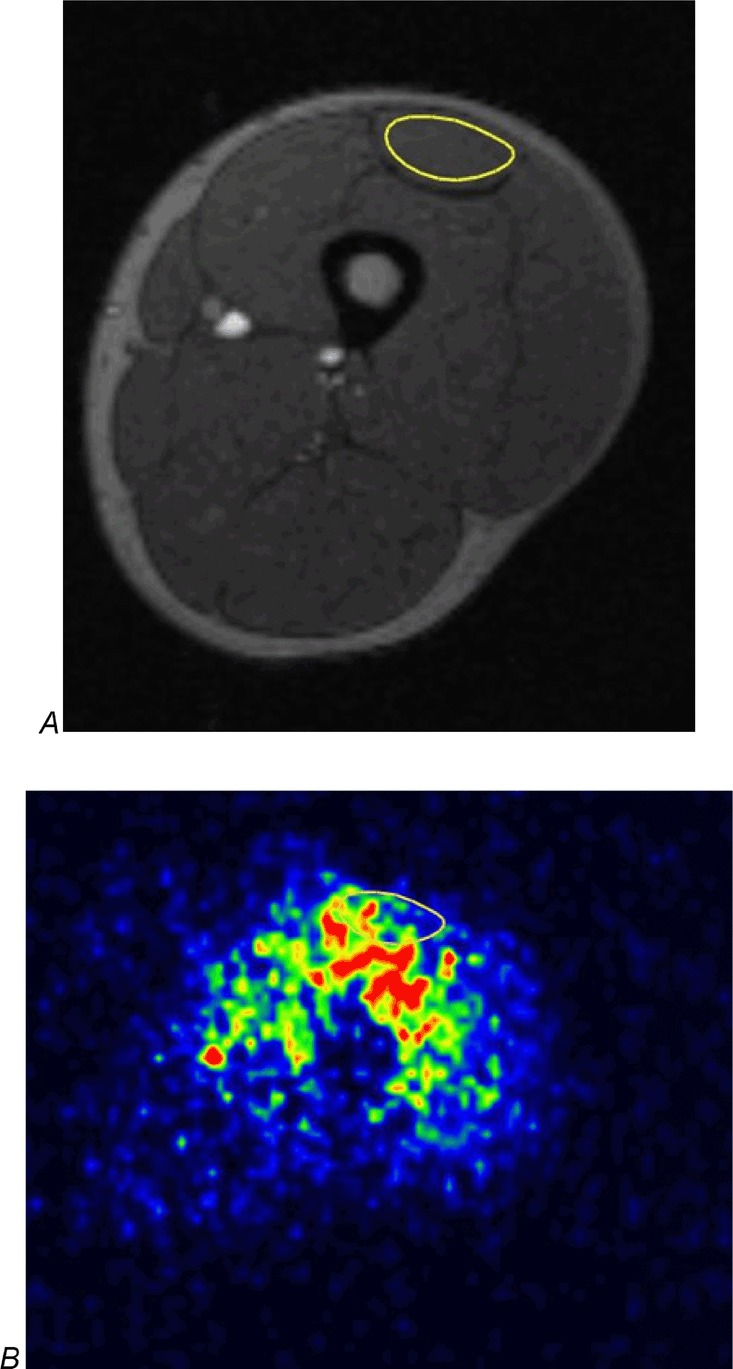
Transaxial MRI and PET images at mid-thigh level A, cross-sectional magnetic resonance imaging (MRI) and B, corresponding parametric blood flow imaging of the femoral region in the same subject. The regions of interest (RoIs) used for analysis were drawn around the quadriceps femoris muscles and the knee flexor muscles. The RoI of the rectus femoris is shown as an example. Red denotes the greatest signal intensity (greatest blood flow) followed by yellow, green and blue.
The relative dispersion (s.d./mean × 100%) of blood flow values in PET image voxels was calculated as an index of spatial flow heterogeneity within the muscles (Fig. 3).
Figure 3.
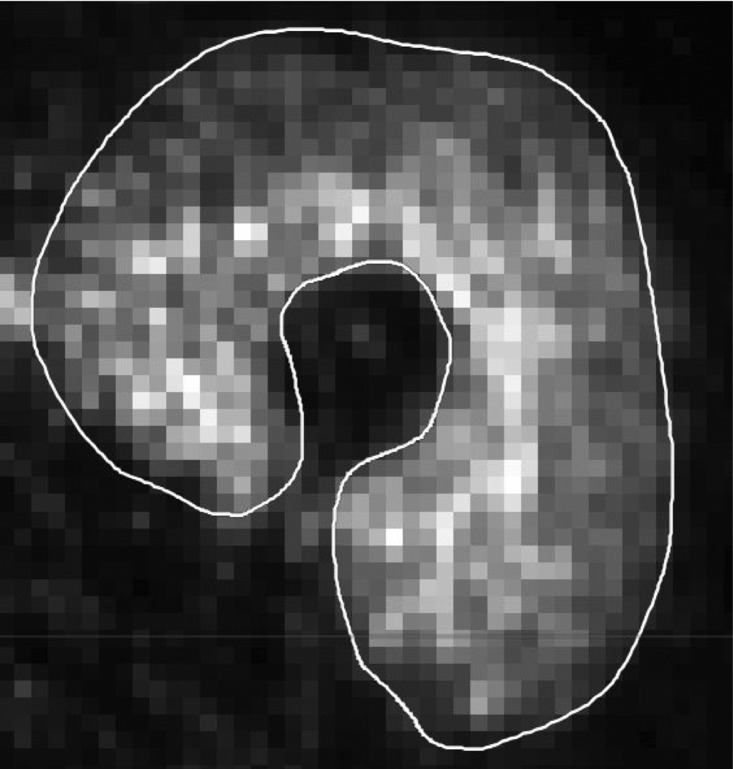
Blood flow heterogeneity in the quadriceps femoris muscle during a fatigue task Voxel-by-voxel map of relative blood flow with a region of interest shown in the exercising quadriceps femoris muscle of one subject. To calculate blood flow heterogeneity, the s.d. of blood flow values for each pixel within each muscle were divided by the mean blood flow for each quadriceps femoris muscle and multiplied by 100. Note the small voxels that show the blood flow level in small pieces of muscle and which are used for perfusion heterogeneity calculations.
Muscle volume
Using Analyze Version 11.0, MRI images were uploaded and areas of bone, fat, muscle and large blood vessels were segmented as previously described (Tanoli et al. 2004; Bashir et al. 2008). Operator-defined threshold signal intensities were assigned to each compartment and only muscle was included in the muscle volume. On the MRI images, four knee extensor muscles were identified and the trace tool was used to draw RoIs along the borders of the rectus femoris, vastus lateralis, vastus medialis and vastus intermedius on every transaxial slice in which these muscles were identifiable. Analyze Version 11.0 was used to export a muscle volume value, which is determined by multiplying cross-sectional area by slice thickness and summing all values for each muscle (Fig. 4). Muscle volumes of the left knee extensors were converted from cubic centimetres into weight (g) by multiplying by an estimated density of 1.04 g ml−1 to calculate total muscle blood flow (ml min−1) (Snyder et al. 1974).
Figure 4.
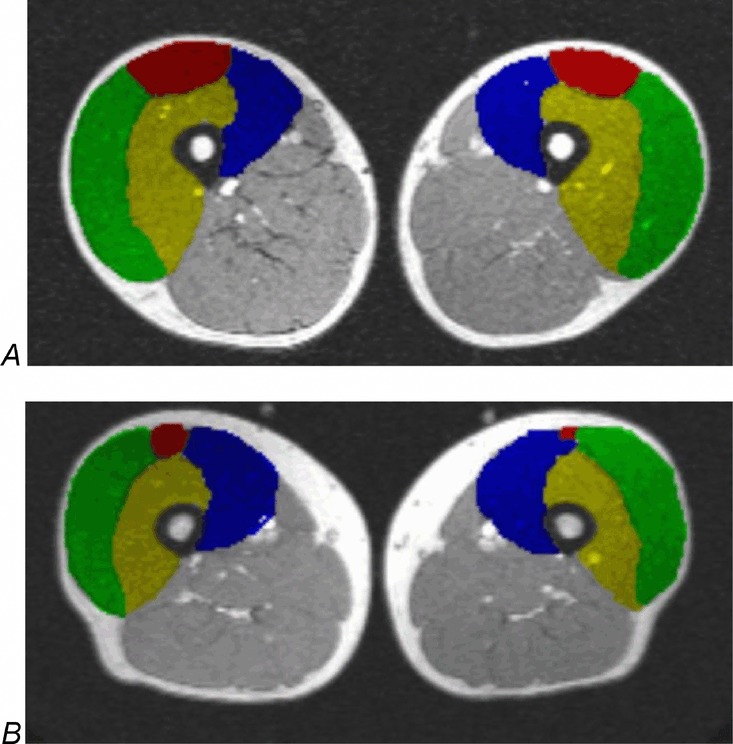
Magnetic resonance imaging of the left and right legs On magnetic resonance imaging, four knee extensor muscles were identified and regions of interest (RoIs) were drawn along the borders of the rectus femoris, vastus lateralis, vastus medialis and vastus intermedius on every transaxial slice on which these muscles were identifiable; RoIs are shown in A, a young man and B, an old man. Analyze 11.0 was used to export muscle volume values, which are determined by multiplying the cross-sectional area by slice thickness and summing all values for each muscle. Green, vastus lateralis; red, rectus femoris; yellow, vastus intermedius; blue, vastus medialis.
Electromyography
Electromyography (EMG) signals were recorded during the two fatiguing contractions with bipolar surface electrodes (Ag-AgCl, 8-mm diameter; 20-mm distance between electrodes) placed over three agonist muscles (rectus femoris, vastus medialis, vastus lateralis) and one antagonist muscle (short head of biceps femoris) using the Telemyo 2400 T G2 (Noraxon USA, Inc.). The electrodes were attached to the skin based on established landmarks between the innervation zone and the end of the tendon. The EMG signals were amplified (×2000), band-pass filtered (13–1000 Hz) and recorded on a computer (2000 samples/s).
The maximal EMG signal for the knee extensor muscles was calculated as the average amplitude over a 0.5 s interval about the peak MVC force. The maximal EMG for the biceps femoris was calculated as the average value over a 0.5 s interval about the peak rectified EMG during maximal knee flexor activity. The maximal EMGs were recorded for each experimental set-up prior to the fatiguing contraction. EMG activity during fatiguing contractions was quantified by averaging the rectified EMG (aEMG) over the first and last 30 s of endurance time and over 15 s intervals centred on the 20%, 40%, 60% and 80% time-points. EMG values were normalized to the aEMG obtained during the MVC. Muscle coactivation was quantified from the EMG measurements (Falconer & Winter, 1985): (2 × antagonist aEMG/agonist aEMG + antagonist aEMG).
Statistical analysis
The dependent variables included: endurance time for the position task; MVC forces; average blood flow and blood flow heterogeneity values in the selected muscles, and EMG activity of selected knee extensor and flexor muscles. Assessments with the Kolmogorov–Smirnov test confirmed that the distributions were normal. A two-factor, repeated-measures ANOVA (task × age) was used to compare MVC forces and endurance times for the force and position tasks (first experiment) between young and old men. Independent t tests were used to analyse MVC forces and endurance times for the position task. A three-factor, repeated-measures ANOVA (muscle × leg × age) was used to compare muscle volumes of left and right knee extensors between young and old men. A four-factor ANOVA (task × age × time × muscle) with repeated measures on time was used to compare changes in blood flow, blood flow heterogeneity, EMG activity and coactivation ratios. Changes in MVC force were examined with a two-factor, repeated-measures ANOVA (task × age). After a significant F-test, pairwise differences were identified using paired and unpaired t tests with Bonferroni corrections as post hoc tests. The significance level was set at P < 0.05. Statistical analyses were performed using spss Version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are reported as the mean ± s.d. within the text and tables and displayed as the mean ± s.e.m. in figures.
Results
Young and old men demonstrated similar MVC forces (418 ± 118 N and 380 ± 79 N, respectively; P = 0.5) and declines in MVC forces after the fatiguing contractions (Table 1). There was no difference between the groups in endurance time on the initial position task (young men: 1330 ± 48 s; old men: 1359 ± 21 s; P = 0.4). Accordingly, the young and old men performed the subsequent fatiguing contractions for similar durations (1209 ± 44 s and 1235 ± 19 s, respectively; P > 0.5). Muscle volumes of knee extensors were greater in the young men than in the old men (P = 0.004) (Table 2). There were no differences in aEMG activity, mean blood flow or blood flow heterogeneity between force and position tasks in either group (task × muscle × age, P > 0.4); accordingly, data were collapsed across force and position tasks for young and old men.
Table 1.
Maximal voluntary contraction (MVC) forces (N) for the knee extensors of young and old men before and after the force and position tasks
| Position task | Force (90%) | Position (90%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Before (N) | After (N) | % Δ | Before (N) | After (N) | % Δ | Before (N) | After (N) | % Δ |
| Young men | 418 ± 75 | 313 ± 65* | −25 ± 12 | 424 ± 80 | 340 ± 107* | −20 ± 7 | 413 ± 70 | 318 ± 52* | −23 ± 2 |
| Old men | 379 ± 110 | 277 ± 105* | −27 ± 10 | 385 ± 65 | 304 ± 56* | −21 ± 4 | 374 ± 62 | 284 ± 59* | −24 ± 4 |
Mean ± s.d. for MVC forces (N) of young and old men performed before and after the force and position tasks during the three sessions: the initial endurance position task and the subsequent force and position tasks for duration equal to 90% of endurance time for the position task. Young and old men demonstrated similar MVC forces and declines in MVC forces after the fatiguing contractions.
P < 0.05 compared with before MVCs.
Table 2.
Muscle volumes measured by magnetic resonance imaging (MRI) of left (exercising) and right (resting) knee extensors in young and old men
| Young men, muscle volume, cm3, mean ± s.d. | Old men, muscle volume, cm3, mean ± s.d. | |||
|---|---|---|---|---|
| Left leg | Right leg | Left leg | Right leg | |
| Knee extensors | 1211 ± 107† | 1194 ± 136† | 848 ± 213 | 844 ± 177 |
| Rectus femoris | 198 ± 15* | 192 ± 32* | 110 ± 69 | 129 ± 47 |
| Vastus lateralis | 352 ± 75* | 374 ± 62* | 231 ± 44 | 294 ± 50 |
| Vastus medialis | 360 ± 75* | 357 ± 79* | 283 ± 98 | 224 ± 70 |
| Vastus intermedius | 300 ± 57* | 271 ± 57* | 223 ± 56 | 196 ± 47 |
Muscle volumes were greater in young men than in old men.
P < 0.05 and †P < 0.01 between young and old men.
Blood flow in knee extensors
When collapsed across tasks and time, a leg main effect in young (P < 0.001) and old (P < 0.001) men indicated that blood flow was greater in the left (exercising) knee extensors than in the right (resting) knee extensors (young men: 8.23 ± 3.46 ml min−1 (100 g)−1 and 2.23 ± 1.16 ml min−1 (100 g)−1, respectively; old men: 11.76 ± 6.65 ml min−1 (100 g)−1 and 1.16 ± 2.07 ml min−1 (100 g)−1, respectively).
In the exercising knee extensors, blood flow increased from the beginning to the end of the task across tasks, groups and muscles (8.12 ± 4.23 ml min−1 (100 g)−1 and 11.69 ± 6.03 ml min−1 (100 g)−1; time main effect, P = 0.001) (Fig. 5) (Table 3A). Across tasks and times, blood flow in the left knee extensor muscles was greater in the old men (12.5 ± 6.2 ml min−1 (100 g)−1) than in the young men (8.6 ± 3.6 ml min−1 (100 g)−1) (age main effect, P < 0.001). Total muscle blood flow in left knee extensors across tasks and times was similar in young and old men (young men: 25.8 ± 12.6 ml min−1 (100 g)−1; old men: 25.1 ± 15.4 ml min−1 (100 g)−1; age main effect, P = 0.77) (Table 3B). In individual muscle components of knee extensors in the exercising leg, blood flow per gram of muscle was greater in old than in young men in the vastus intermedius and medialis muscles at the beginning and end across tasks (muscle × age × time, P = 0.03) (Table 3A), but no differences were observed in total muscle blood flow (Table 3B). In the exercising leg, greater blood flow was observed in the vastus intermedius and vastus medialis in old men and in the vastus intermedius in young men compared with the other two and three knee extensor muscles, respectively, across tasks and times (muscle × age, P = 0.03) (Fig. 6A).
Figure 5.
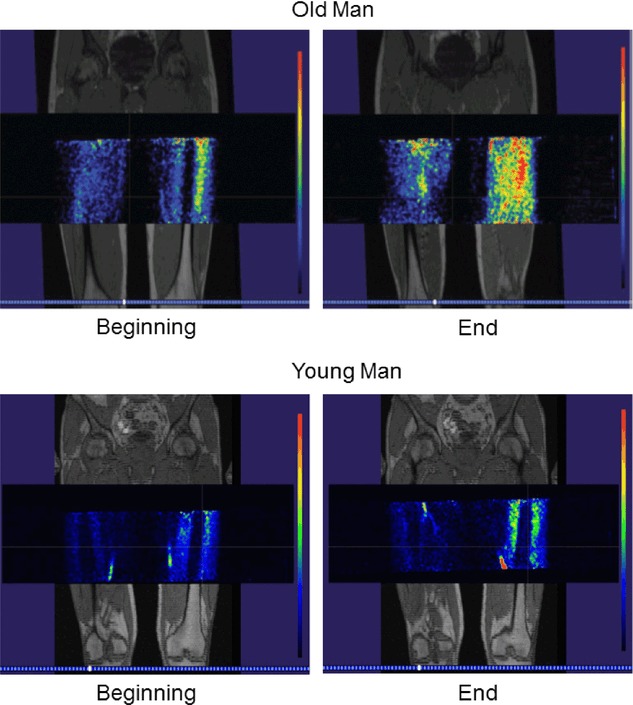
Fused MRI and PET images Fused magnetic resonance imaging and positron emission tomography in a young and an old man at the beginning and end of one fatiguing contraction. Red denotes the greatest blood flow, followed by yellow, green and blue, respectively.
Table 3.
A, Blood flow per unit mass of muscle (ml min−1 (100 g)−1) and B, total muscle blood flow (ml min−1) in the exercising (left) knee extensor muscles at the beginning and end of fatiguing contractions across force and position tasks
| Beginning, blood flow, ml min−1 (100 g)−1, mean ± s.d. | End, blood flow, ml min−1 (100 g)−1, mean ± s.d. | |||
|---|---|---|---|---|
| Young men | Old men | Young men | Old men | |
| A | ||||
| Knee extensors | 6.64 ± 2.03 | 9.76 ± 5.34† | 9.83 ± 3.86§ | 13.76 ± 7.27†§ |
| Rectus femoris | 6.33 ± 2.41 | 7.90 ± 6.02 | 9.09 ± 3.49 | 12.21 ± 7.61 |
| Vastus lateralis | 6.21 ± 1.96 | 8.77 ± 6.27 | 9.77 ± 3.82‡ | 11.12 ± 7.33 |
| Vastus medialis | 5.66 ± 1.38 | 8.08 ± 2.6* | 7.80 ± 3.00 | 12.13 ± 5.11* |
| Vastus intermedius | 8.37 ± 1.29 | 14.27 ± 3.32† | 12.66 ± 3.82§ | 19.59 ± 6.44*‡ |
| B | ||||
| Knee extensors | 83.9 ± 17.9 | 82.5 ± 22.8 | 124.3 ± 39.5‡ | 116.6 ± 35.2‡ |
| Rectus femoris | 13.1 ± 5.3 | 7.6 ± 5.5 | 18.9 ± 8.0 | 13.4 ± 11.6 |
| Vastus lateralis | 22.5 ± 7.5 | 20.6 ± 13.8 | 35.2 ± 12.9‡ | 26.4 ± 17.5 |
| Vastus medialis | 21.6 ± 7.1 | 23.1 ± 10.5 | 29.8 ± 13.9 | 33.0 ± 10.6 |
| Vastus intermedius | 26.2 ± 6.6 | 32.5 ± 7.4 | 39.7 ± 13.5‡ | 44.0 ± 9.9‡ |
Muscle volumes of the left extensors were converted from cm3 into weight (g) by multiplying by an estimated density of 1.04 g ml−1 to calculate total muscle blood flow (ml min−1).
P < 0.05 and
P < 0.01 between young and old men.
P < 0.05 and
P < 0.01 between the beginning and end of tasks within a group.
Figure 6.
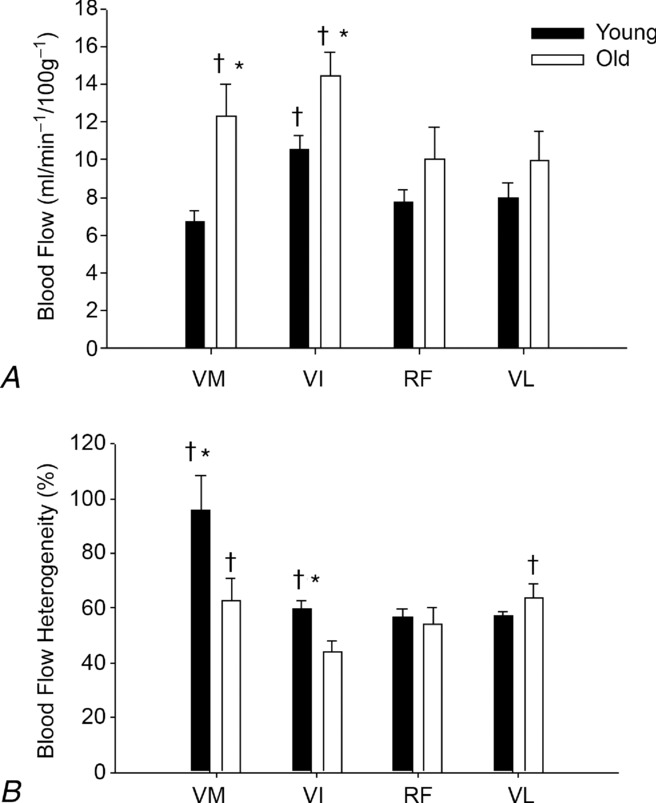
Mean blood flow and blood flow heterogeneity across tasks and times
In the knee extensors of the resting leg, a time main effect (P = 0.04) indicated that blood flow increased from the beginning (1.95 ± 1.18 ml min−1 (100 g)−1) to the end (2.36 ± 1.38 ml min−1 (100 g)−1) of the tasks, across tasks, muscles and groups. Blood flow in resting knee extensors was similar in young and old men (age main effect, P = 0.3).
Blood flow in knee flexors
In the knee flexors of the exercising leg, blood flow was greatest in the short head of the biceps femoris (4.16 ± 2.64 ml min−1 (100 g)−1) across time, tasks and groups (long head of the biceps femoris: 1.47 ± 0.64 ml min−1 (100 g)−1; semimembranosus: 1.47 ± 0.82 ml min−1 (100 g)−1; semitendinosus: 1.39 ± 1.01 ml min−1 (100 g)−1; muscle main effect, P < 0.001). An age main effect (P = 0.04) indicated that blood flow was greater in the knee flexors of old men (2.42 ± 2.53 ml min−1 (100 g)−1) than in those of young men (1.85 ± 1.00 ml min−1 (100 g)−1) across muscles, time and tasks.
Blood flow in the knee flexors of the resting leg was similar in young and old men (age main effect, P = 0.08) and increased from the beginning to end of the task across tasks, muscles and groups (1.65 ± 0.89 ml min−1 (100 g)−1 and 2.07 ± 1.14 ml min−1 (100 g)−1, respectively; time main effect, P = 0.01).
Blood flow heterogeneity in knee extensors
Collapsed across tasks, a leg × time interaction (P < 0.05) indicated that blood flow heterogeneity in the extensors was greater in the resting leg than in the exercising leg at the beginning (89 ± 30% and 67 ± 38%, respectively) and end (91 ± 31% and 57 ± 21%, respectively) of the task.
The heterogeneity of blood flow in the exercising quadriceps femoris muscles decreased from the beginning to end of the task across tasks and groups (67 ± 38% and 57 ± 21%, respectively). Collapsed across tasks, time and muscles, an age main effect in the exercising knee extensors indicated greater heterogeneity in the young (67 ± 34%) than the old (56 ± 27%) men. Collapsed across tasks and times, flow was more heterogeneous in young men than in old men in the exercising vastus medialis (96 ± 56% and 63 ± 35%, respectively; P < 0.05) and vastus intermedius (60 ± 15% and 44 ± 19%, respectively; P < 0.005) (muscle × age) (Fig. 6B). In the young men, the greatest heterogeneity in the exercising leg was observed in the vastus medialis (96 ± 56%) compared with that in the vastus lateralis (57 ± 5%), rectus femoris (56 ± 12%) and vastus intermedius (60 ± 15%) (P < 0.05) (Fig. 6B). In the old men, the heterogeneity of blood flow in the exercising vastus lateralis (64 ± 23%) and vastus medialis (63 ± 35%) was greater than that in the rectus femoris (54 ± 25%) and vastus intermedius (44 ± 19%) (P < 0.05) (Fig. 6B).
In resting knee extensors, blood flow heterogeneity across tasks, time and muscles was similar in young and old men (age main effect, P = 0.79).
Blood flow heterogeneity in knee flexors
Across tasks and groups, a time main effect (P = 0.002) in resting knee flexors indicated that heterogeneity decreased from the beginning (101 ± 31%) to end (96 ± 25%) of the task. Blood flow heterogeneity in resting knee flexors was similar in young and old men across tasks, times and muscles (age main effect, P = 0.52). A muscle main effect (P = 0.008) in the exercising leg indicated blood flow was least heterogeneous in the short head of the biceps femoris (79 ± 15%) compared with that in the other knee flexors (long head of the biceps femoris: 104 ± 33%; semimembranosus: 113 ± 38%; semitendinosus: 118 ± 38%) across time, tasks and groups.
EMG amplitude
The aEMG for the agonist muscles (rectus femoris, vastus lateralis, vastus medialis) increased similarly in both groups during the two types of fatiguing contraction (task × muscle × age, P = 0.89), but at a greater rate in the younger men (time × age, P < 0.001) (Fig. 7). The aEMG for the knee extensor muscles collapsed across tasks increased from 15.1 ± 6.1% to 39.9 ± 14.8% MVC in the young men and from 15.0 ± 6.1% to 28.3 ± 10.7% MVC in the old men. The aEMG for the knee extensor muscles was similar at the start and at 20% and 40% of endurance time in the two groups, but was greater in the young men at 60%, 80% and 100% of endurance time (muscle × time × age, P = 0.008).
Figure 7.
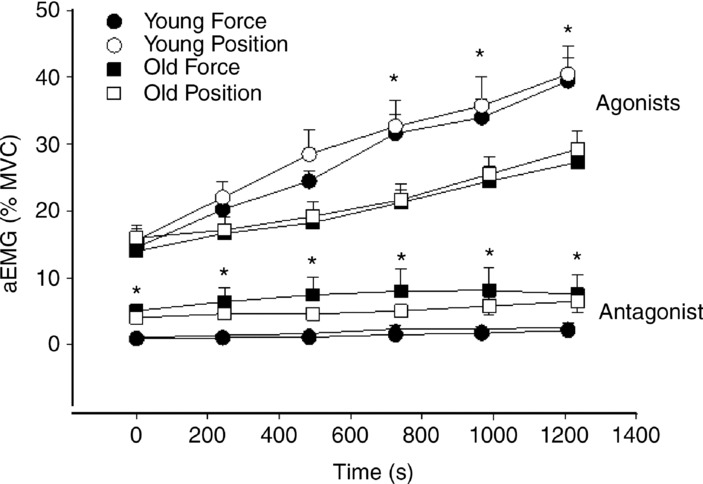
Change in electromyography amplitude during fatiguing contractions
The aEMG for the antagonist muscle (short head of the biceps femoris) increased similarly during the two fatiguing contractions in both groups of subjects (time × age, P = 0.07) (Fig. 7). An age main effect (P = 0.04) indicated that aEMG for the antagonist muscle was greater at each time-point in the old men than in the young men.
The coactivation ratios did not change during the sustained contractions and were comparable between tasks at each time-point in the young men (force task, start: 0.12 ± 0.06, end 0.10 ± 0.05; position task, start: 0.13 ± 0.06, end: 0.12 ± 0.05) and the old men (force task, start: 0.53 ± 0.16, end 0.43 ± 0.2; position task, start: 0.40 ± 0.15, end: 0.36 ± 0.21) (task × age × time, P = 0.41). However, there was a significant main effect for age (P < 0.001) as a result of greater coactivation ratios in the old men in both the force and position tasks.
Discussion
The results of the present study provide novel information about blood flow to the knee muscles in young and old men during two types of fatiguing isometric knee extension contraction maintained for similar durations with similar levels of target force. During both the force and position tasks, blood flow per unit mass of muscle was greater and less heterogeneous in the active knee extensor muscles in old than in young men; this is likely to be related to alterations in muscle fibre type characteristics that occur during ageing (Petrofsky & Lind, 1975; Larsson & Karlsson, 1978; Clarkson et al. 1981). The passive knee flexor muscles of old men also exhibited greater and less heterogeneous blood flow per unit mass of muscle compared with those of young men during both tasks. In combination, the results suggest that despite an age-induced loss of muscle volume, exercise performance and muscle blood flow can be sustained effectively by maintaining a physically active lifestyle until, at the least, the age of almost 80 years.
Blood flow is the most important factor regulating the oxygen supply of the skeletal muscles, especially during exercise. Limb blood flow can be measured in humans using techniques such as plethysmography, thermodilution and Doppler ultrasound (McCully & Posner, 1995). Studies using these techniques have suggested that blood flow and vascular conductance during exercise is reduced (Proctor et al. 1998; Dinenno et al. 1999) or unchanged (Jasperse et al. 1994; Magnusson et al. 1994) by ageing. However, the techniques used in these studies provided information about whole limb flow only, which includes contributions from bone, skin and fat. Recent MRI blood flow investigations have provided information directly from muscle tissue (Wray et al. 2009; Layec et al. 2013) and it seems that when non-muscular tissues are excluded and the lower muscle volumes of older subjects are taken into account, the age-induced differences in blood flow disappear, especially in muscles at rest and during low-intensity exercise (Lawrenson et al. 2003; Donato et al. 2006).
Explanations for the greater blood flow per unit mass of muscle in the older men may refer to increased stiffness of the blood vessels (Bailey, 2001) and tendons (Kjaer, 2004) and changes in the extracellular matrix (Kjaer, 2004; Kragstrup et al. 2011) that may impair direct force transmission to the muscle and thus the efficiency of performance in the fatiguing tasks applied. However, the present findings also suggest that the smaller knee extensor muscles in older men required more oxygen and nutrients per gram of muscle than in the younger men in order to maintain a similar external load with the knee extensors. This is in line with the findings of a previous study (Rudroff et al. 2013), which showed increased energy requirements in the form of greater [18F]-2-fluoro-2-deoxy-D-glucose ([18F]-FDG) uptake by knee muscles of older men compared with knee muscles of younger men despite the application of lower target forces during a similar experimental protocol. Furthermore, whereas younger adults are likely to have modulated the level of presynaptic inhibition of Ia afferents across force and position control tasks (Baudry et al. 2010), older adults preferred to increase the amount of antagonist coactivation as EMG coactivation indices were higher in older men than in younger men during both tasks. The finding of greater [18F]-FDG uptake in the knee extensors, knee flexors and hip muscles of the old men after the fatigue tasks further supports the preference for this strategy. This study advances these findings by showing that blood flow is increased in all these muscles, which implies that the old men used a less efficient strategy to perform essentially the same fatiguing tasks as the young men. However, when we calculated total muscle blood flow (blood flow per unit mass of muscle multiplied by muscle mass), the age-related differences essentially disappeared and total blood flow in the whole muscle was similar in both groups. Importantly, Mortensen et al. (2012) recently showed that whole limb blood flow is reduced in healthy inactive old men, but maintained in physically active old men. These findings strongly align with those of the present study, which included a cohort of physically active old men. Furthermore, our findings are also in line with those of previous studies that directly measured muscle blood flow. For example, the findings of Kalliokoski et al. (2003b) support our finding of an increase in muscle blood flow during low-intensity isometric exercise. However, in the previous study, the increase in blood flow was associated with greater blood flow heterogeneity, whereas the present study demonstrated a decrease in blood flow heterogeneity and a corresponding increase in mean blood flow.
Another significant and novel finding in the present study is that blood flow within the knee extensor muscles of old men was less heterogeneous. The most likely explanation for this finding refers to the shift to a less heterogeneous type of muscle tissue with increased age caused by either a conversion from type II to type I muscle fibres or the preferential loss of type II muscle fibres (Petrofsky & Lind, 1975; Larsson & Karlsson, 1978; Clarkson et al. 1981). Accordingly, young muscles may demonstrate greater heterogeneity of blood flow because they include a greater proportion of type II muscle fibres, whereas a higher proportion of type I fibres may contribute to a less heterogeneous and enhanced blood flow. Increased force generation by smaller older muscles and associated greater blood flow and less heterogeneity are also consistent with findings of previous studies that showed reduced heterogeneity of glucose uptake and blood flow in human skeletal muscles with increasing exercise intensity (Heinonen et al. 2007, 2012).
In exercising knee extensors, greater mean blood flow and less blood flow heterogeneity were found in the vastus intermedius and vastus medialis of older than younger men. This finding of less heterogeneity in the two exercising extensors with the greatest blood flow (vastus intermedius and vastus medialis) is consistent with previous functional human findings (Kalliokoski et al. 2000), as well as with findings from human biopsy studies showing that blood flow and capillarity are highest in the muscles that contain the greatest proportion of slow oxidative fibres: the vastus medialis and vastus intermedius (Johnson et al. 1973; Edgerton et al. 1975). Muscle blood flow heterogeneity may also have been affected by differences in sympathetic activity and sympatholysis, which have been shown by Parker et al. (2007) to be altered in old men. Future studies are needed to further explore this possible connection.
Despite a similar target torque, the aEMG activity of knee extensors increased at a greater rate in young men than in old men and was greater in young men at 60%, 80% and 100% of endurance time. This finding reveals differences in either recruitment or discharge between the two groups of men. In young adults, the increase in EMG activity in knee extensors during low-force fatiguing contraction largely reflects the recruitment of larger motor units as the muscle becomes fatigued, with a minimal contribution from discharge rate (Mottram et al. 2005; Rudroff et al. 2010). Given the preferential loss of type II muscle fibre with ageing, it may be that the old men experienced a more gradual reduction in the force capacity of the active muscle fibres, which were likely to have greater oxidative capacity. The old men used greater coactivation than the younger men to accomplish the fatiguing contractions, a findings that has also been demonstrated previously (Rudroff et al. 2013).
In regard to the different exercise tasks investigated, no differences between force and position tasks in aEMG activity and blood flow measurements in knee extensors and flexors emerged in either group of subjects. This finding is in line with a previous muscle energetics study (Rudroff et al. 2013) that used a similar experimental set-up and found no differences between force and position tasks in aEMG activity in knee muscles, although glucose uptake was greater in young men during the position task than the force task.
Methodological considerations
The degree of blood flow occlusion during an exercise task plays an important role in muscle fatigue protocols, and could influence comparative muscle fatigability in old and young adults during voluntary contractions. Muscle blood flow occlusion is more severe during isometric than dynamic contractions and is also greater with larger muscle size and greater absolute force production (Bigland-Ritchie et al. 1995). Thus, it is possible that even at this low isometric contraction load of 25% MVC, muscle blood flow may have been compromised (de Ruiter et al. 2007), which may explain the somewhat lower blood flow values reported in this study compared with those of previous studies that have employed Doppler ultrasound and thermodilution methods (Kilbom & Persson 1982; Sjøgaard et al. 1986; Gaffney et al. 1990; Rådegran 1997). However, these studies used different experimental designs (e.g. leg posture, contraction loads, only young adults) and therefore it is difficult to compare their findings directly with those of the present study. Regarding the exercise loads, Kalliokoski et al. (2003b) observed that muscle perfusion may be hampered even at a load of 10% MVC. In this respect, the recent findings of significantly decreased post-exercise muscle perfusion with modest (∼37 mmHg) compression from outside the thigh via compression shorts (Sperlich et al. 2013) further support the suggestion that muscle perfusion may have been compromised in the present task protocols. However, as MVC and thus the relative exercise loads were comparable between the young and old men, blood flow occlusion should have occurred similarly in both groups and hence should not affect our main results. Nonetheless, the lower blood flow per unit mass of muscle identified in the younger subjects may partly reflect occlusion as a result of their larger muscle mass (Bigland-Ritchie et al. 1995). Similar MVC but larger muscle mass in the younger subjects supports the suggestions of previous studies (Reed et al. 1991; Beliaeff et al. 2008; Clark & Manini 2008; Manini & Clark 2012) that muscle mass and muscle strength are only weakly related in the healthy older population. A major strength of this study was its ability to determine muscle volume in the four knee extensor muscles with the exclusion of fat tissue and large blood vessels. Furthermore, the old men in this study were more physically active than is typical of men of this age, which suggests that physical activity is the key component to maintaining an intact neural drive to muscles during MVCs in older adults (Enoka, 1997). Similar declines in MVC force after the fatiguing contractions indicate similar motivation in the young and old men.
In conclusion, the findings of the present study demonstrate that smaller knee extensor muscles in old men required greater blood flow per gram of muscle to accomplish fatiguing contractions with knee extensor muscles for similar times and with similar torques as young men. Although oxygen consumption and extraction were not measured in this study, it is suggested that older men performing the same tasks as young men consume more oxygen and nutrients per gram of muscle than do their younger counterparts. However, future studies are necessary to investigate this possibility. We suggest that the greater proportion of muscle fibre type I in old muscles supports the ability to sustain the contraction, resulting in greater blood flow and less heterogeneity of blood flow. Furthermore, these findings indicate that physically active old men have intact neural drive to muscle and achieve adequate exercise hyperaemia despite their smaller muscle volume. Although some studies suggest the presence of greater fatigue resistance in older adults as a result of the increased metabolic economy of older skeletal muscle (Kent-Braun et al. 2002; Lanza et al. 2007; Kent-Braun, 2009), the findings of the present study suggest the contrary and imply that sustained physical activity is the key factor in explaining the similarities in maximal force production and fatigue resistance in old and young men.
Acknowledgments
The authors thank Sergey V. Nesterov for his contributions to the imaging software (Carimas) and R. M. Enoka for his support during the grant application.
Competing interests
None declared.
Author contributions
T.R. and K.K.K. conceived the conceptual framework of the study, designed and performed the experiments. J.A.W., M.B., M.S., K.K. contributed to the data analyses. T.R. wrote the manuscript. K.K.K. and I.H. contributed to the interpretation of data. All authors have read and approved the final manuscript for submission.
Funding
This work was supported by an award by the National Institute on Aging (AG33744) to T.R.
References
- Allman BL, Rice CL. Incomplete recovery of voluntary isometric force after fatigue is not affected by old age. Muscle Nerve. 2001;24:1156–1167. doi: 10.1002/mus.1127. [DOI] [PubMed] [Google Scholar]
- Allman BL, Rice CL. Neuromuscular fatigue and aging: central and peripheral factors. Muscle Nerve. 2002;25:785–796. doi: 10.1002/mus.10116. [DOI] [PubMed] [Google Scholar]
- Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol. 2010;103:623–631. doi: 10.1152/jn.00839.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir A, Laciniy E, Lassa-Claxton S, Yarasheski KE. Magnetic resonance imaging for quantifying regional adipose tissue content in HIV-infected people at risk for developing cardiometabolic syndrome. J Cardiometab Syndr. 2008;3:115–118. doi: 10.1111/j.1559-4572.2008.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliaeff S, Bouchard DR, Hautier C, Brochu M, Dionne IJ. Association between muscle mass and isometric muscle strength in well-functioning older men and women. J Aging Phys Act. 2008;16:484–493. doi: 10.1123/japa.16.4.484. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Rice CL, Garland SJ, Walsh ML. Task-dependent factors in fatigue of human voluntary contractions. Adv Exp Med Biol. 1995;384:361–380. doi: 10.1007/978-1-4899-1016-5_29. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Henderson TK, Nolta BE, Pursley PJ, Sandfort GL. Effect of aging on fatigue characteristics of elbow flexor muscles during sustained submaximal contraction. J Appl Physiol. 2011;91:2654–2664. doi: 10.1152/jappl.2001.91.6.2654. [DOI] [PubMed] [Google Scholar]
- Clark BS, Manini TM. Sarcopenia ≠ dynapenia. J Gerontol. 2008;63a:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Kroll W, Melchionda AM. Age, isometric strength, rate of tension development and fibre type composition. J Gerontol. 1981;36:648–653. doi: 10.1093/geronj/36.6.648. [DOI] [PubMed] [Google Scholar]
- Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol. 1955;128:258–267. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H. Age-related changes in the microcirculation of skeletal muscle. Adv Exp Med Biol. 1998;454:343–348. doi: 10.1007/978-1-4615-4863-8_40. [DOI] [PubMed] [Google Scholar]
- De Ruiter CJ, Goudsmit JFA, van Tricht JA, de Haan A. The isometric torque at which knee-extensor muscle reoxygenation stops. Med Sci Sports Exerc. 2007;39:443–452. doi: 10.1249/mss.0b013e31802dd3cc. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans – relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790. [PubMed] [Google Scholar]
- Doherty TJ. Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Neural adaptations with chronic physical activity. J Biomech. 1997;30:447–455. doi: 10.1016/s0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Baudry S, Rudroff T, Farina D, Klass B, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol. 2011;21:208–219. doi: 10.1016/j.jelekin.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol. 1985;25:135–149. [PubMed] [Google Scholar]
- Gaffney FA, Sjøgaard G, Saltin B. Cardiovascular and metabolic responses to static contraction in man. Acta Physiol Scand. 1990;138:249–258. doi: 10.1111/j.1748-1716.1990.tb08844.x. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Nesterov S, Kemppainen J, Fujimoto T, Knuuti J, Kalliokoski KK. Increasing exercising intensity reduces heterogeneity of glucose uptake in human skeletal muscles. PLOS One. 2012;7:1–7. doi: 10.1371/journal.pone.0052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, Oikonen V, Nuutila P, Knuuti J, Hellsten Y, Boushel R, Kalliokoski KK. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol. 2010;108:378–386. doi: 10.1152/japplphysiol.00745.2009. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol. 2007;103:2042–2048. doi: 10.1152/japplphysiol.00567.2007. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Appl Physiol. 2002;88:3087–3096. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol. 2004;97:1723–1732. doi: 10.1152/japplphysiol.00460.2004. [DOI] [PubMed] [Google Scholar]
- Iida H, Kanno I, Miura S, Murakami M, Takahashi K, Uemura K. Error analysis of a quantitative cerebral blood flow measurement using H215O autoradiography and positron emission tomography, with respect to the dispersion of the input function. J Cereb Blood Flow Metab. 1986;6:536–545. doi: 10.1038/jcbfm.1986.99. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol. 1994;474:353–360. doi: 10.1113/jphysiol.1994.sp020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Kemppainen J, Larmola K, Takala TO, Peltoniemi P, Oksanen A, Ruotsalainen U, Cobelli C, Knuuti J, Nuutila P. Blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur J Appl Physiol. 2000;83:395–401. doi: 10.1007/s004210000267. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Oikonen V, Takala TO, Sipilä H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001;280:E1015–E1021. doi: 10.1152/ajpendo.2001.280.6.E1015. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Laaksonen MS, Knuuti J, Nuutila P. Perfusion distribution between and within muscles during intermittent static exercise in endurance-trained and untrained men. Int J Sports Med. 2003a;24:400–403. doi: 10.1055/s-2003-41179. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Laaksonen MS, Takala TO, Knuuti J, Nuutila P. Muscle oxygen extraction and perfusion heterogeneity during continuous and intermittent static exercise. J Appl Physiol. 2003b;94:953–958. doi: 10.1152/japplphysiol.00731.2002. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Scheede-Bergdahl C, Kjaer M, Boushel R. Muscle perfusion and metabolic heterogeneity: insights from non-invasive imaging techniques. Exerc Sport Sci Rev. 2006;34:164–170. doi: 10.1249/01.jes.0000240018.07502.48. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage. Exerc Sport Sci Rev. 2009;37:3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibom A, Persson J. Leg blood flow during static exercise. Eur J Appl Physiol. 1982;48:367–377. doi: 10.1007/BF00430227. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011;21:749–757. doi: 10.1111/j.1600-0838.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583:1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand. 1978;104:129–136. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- Layec G, Haseler LJ, Richardson RS. Reduced muscle oxidative capacity is independent of O2 availability in elderly people. Age. 2013;35:1183–1192. doi: 10.1007/s11357-012-9442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Isberg B, Saltin B. Cardiovascular responses during one- and two-legged exercise in middle-aged men. Acta Physiol Scand. 1994;150:353–362. doi: 10.1111/j.1748-1716.1994.tb09699.x. [DOI] [PubMed] [Google Scholar]
- Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KK, Posner JD. The application of blood flow measurements to the study of aging muscle. J Gerontology. 1995;50:130–136. doi: 10.1093/gerona/50a.special_issue.130. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the aging human leg. J Physiol. 2012;23:6227–6236. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol. 2005;93:1381–1392. doi: 10.1152/jn.00837.2004. [DOI] [PubMed] [Google Scholar]
- Nesterov SV, Han C, Mäki M, Kajander S, Naum AG, Helenius H, Lisinen I, Ukkonen H, Pietilä M, Joutsiniemi E, Knuuti J. Myocardial perfusion quantitation with 15O-labelled water PET: high reproducibility of the new cardiac analysis software (Carimas™) Eur J Nucl Med Mol Imaging. 2009;36:1594–1602. doi: 10.1007/s00259-009-1143-8. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol. 2007;292:H1148–H1156. doi: 10.1152/ajpheart.00729.2006. [DOI] [PubMed] [Google Scholar]
- Petrofsky JS, Lind AR. Aging, isometric strength and endurance, and cardiovascular responses to static effort. J Appl Physiol. 1975;38:91–95. doi: 10.1152/jappl.1975.38.1.91. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol. 2003;94:1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Raitakari M, Nuutila P, Ruotsalainen U, Teras M, Eronen E, Laine H, Raitakari OT, Iida H, Knuuti MJ, Yki-Jarvinen H. Relationship between limb and muscle blood flow in man. J Physiol. 1996;496:543–549. doi: 10.1113/jphysiol.1996.sp021705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RL, Pearlmutter L, Yochum K, Meredith KE, Mooradian AD. The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc. 1991;39:555–561. doi: 10.1111/j.1532-5415.1991.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Jordan K, Enoka JA, Matthews SD, Baudry S, Enoka RM. Discharge of biceps brachii motor units is modulated by load compliance and forearm posture. Exp Brain Res. 2010;202:111–120. doi: 10.1007/s00221-009-2116-7. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Holmes MR, Matthews SD, Enoka RM. Muscle activity and time to task failure differ with load compliance and target force for elbow flexor muscles. J Appl Physiol. 2011;110:125–136. doi: 10.1152/japplphysiol.00605.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T, Kalliokoski KK, Block DE, Gould JR, Klingensmith WC, Enoka RM. PET/CT imaging of age- and task-associated differences in muscle activity during fatiguing contractions. J Appl Physiol. 2013;114:1211–1219. doi: 10.1152/japplphysiol.01439.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen U, Raitakari M, Nuutila P, Oikonen V, Sipila H, Teras M, Knuuti MJ, Bloomfield PM, Iida H. Quantitative blood flow measurement of skeletal muscle using oxygen-15-water and PET. J Nucl Med. 1997;38:314–319. [PubMed] [Google Scholar]
- Sipilae HT, Clark JC, Peltola O, Teraes M. An automatic [15O] H2O production system for heart and brain studies. J Labelled Cpd. Radiopharm. 2001;44 Suppl. 1. [Google Scholar]
- Sjøgaard G, Kiens B, Jørgensen K, Saltin B. Intramuscular pressure, EMG, and blood flow during low-level prolonged static contraction in man. Acta Physiol Scand. 1986;128:475–484. doi: 10.1111/j.1748-1716.1986.tb08002.x. [DOI] [PubMed] [Google Scholar]
- Snyder WS, Cook MJ, Nases ES, Karhausen LR, Parry-Howells G, Tipton IH. International Commission of Radiological Protection No. 23. New York, NY: Pergamon Press; 1974. Report of the task group of the reference man; p. 112. pp. . [Google Scholar]
- Sperlich B, Born DP, Kaskinoro K, Kalliokoski KK, Laaksonen MS. Squeezing the muscle: compression clothing and muscle metabolism during recovery from high intensity exercise. PLOS One. 2013;8:e60923. doi: 10.1371/journal.pone.0060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Mcleod SA. Maximum voluntary contraction in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109. [PubMed] [Google Scholar]
- Tanoli T, Yue P, Yablonsky D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and issue sensitivity. J Lipid Res. 2004;45:941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297:H1870–H1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


