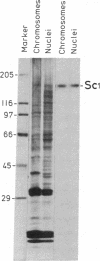
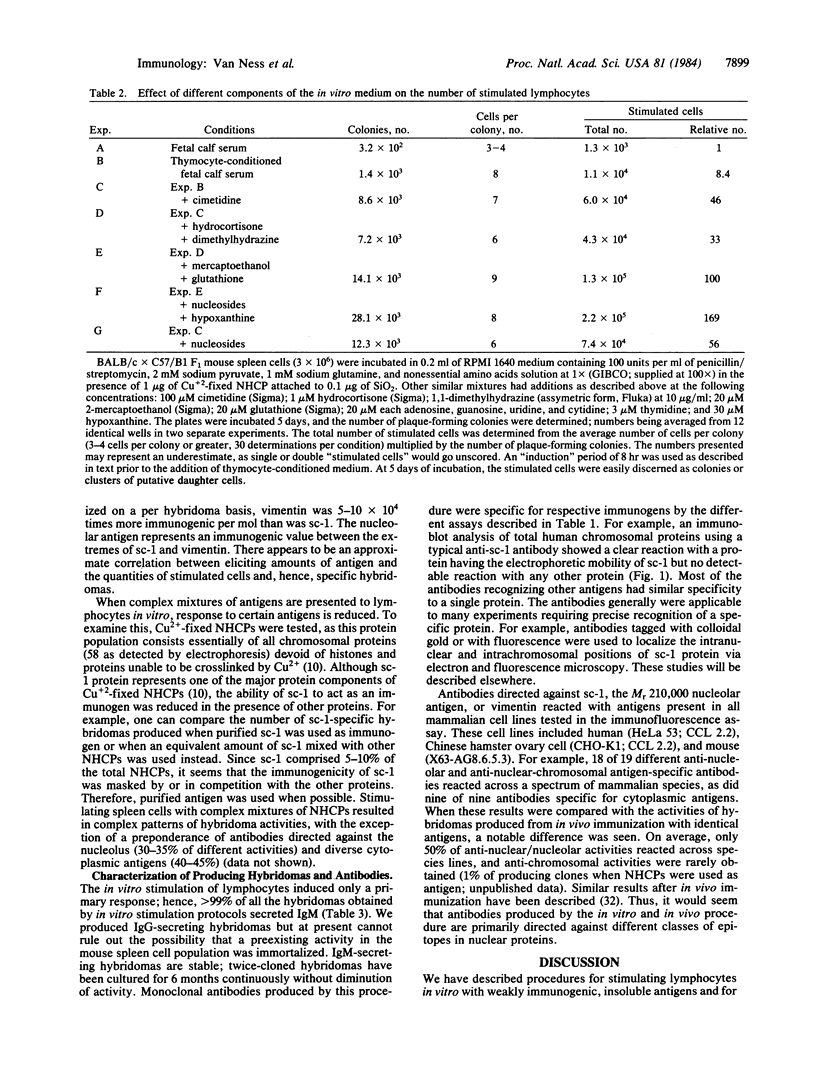
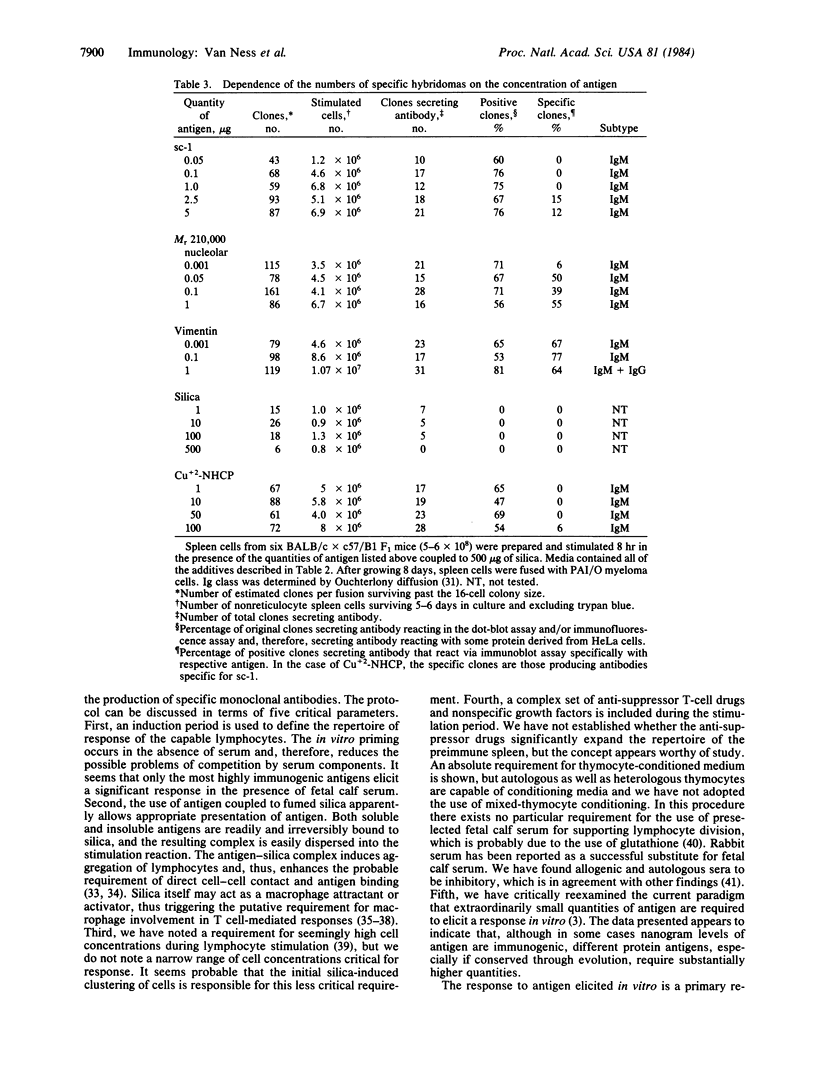
Abstract
A procedure is described for immunizing in vitro and stimulating proliferation of specific B-cell lymphocytes. The method is applicable to production of monoclonal antibodies against proteins that are soluble only in denaturing solvents. An induction period is described in which antigen is presented to the B-cell population in the absence of serum. Also, antigen is coupled to mitogenic silica, which allows the effective presentation of both soluble and insoluble antigens. The results indicate hybridomas can be obtained that secrete IgMs directed against highly conserved or weakly immunogenic antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Lernhardt W., Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell. 1977 Jan;10(1):27–34. doi: 10.1016/0092-8674(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Burger M. A serum-free medium for the Mishell-Dutton system. Eur J Immunol. 1977 Dec;7(12):906–908. doi: 10.1002/eji.1830071216. [DOI] [PubMed] [Google Scholar]
- Burger M. Immunization of mouse spleen cell cultures in the absence of serum and its proteins using SiO2 and 2-mercaptoethanol. Immunology. 1982 Feb;45(2):381–385. [PMC free article] [PubMed] [Google Scholar]
- Burger M. The replacement of serum by SiO2 (aerosil) and 2-mercaptoethanol or diethanoldisulfide in the immunization and stimulation of spleen cell cultures. FEBS Lett. 1980 Sep 8;118(2):212–214. doi: 10.1016/0014-5793(80)80221-3. [DOI] [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Immune responses in vitro. I. Culture conditions for antibody synthesis. Cell Immunol. 1972 Feb;3(2):264–276. doi: 10.1016/0008-8749(72)90165-7. [DOI] [PubMed] [Google Scholar]
- Duclos H., Galanaud P., Maillot M. C., Crevon M. C., Dormont J. Macrophage requirement for the in vitro response to an insolubilized T-independent antigen. Scand J Immunol. 1979;9(2):159–166. doi: 10.1111/j.1365-3083.1979.tb02718.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. Human T lymphocyte antigens as defined by monoclonal antibodies. Immunol Rev. 1981;57:127–161. doi: 10.1111/j.1600-065x.1981.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Hengartner H., Luzzati A. L., Schreier M. Fusion of in vitro immunized lymphoid cells with X63Ag8. Curr Top Microbiol Immunol. 1978;81:92–99. doi: 10.1007/978-3-642-67448-8_14. [DOI] [PubMed] [Google Scholar]
- Hoffeld J. T., Oppenheim J. J. The capacity of fetal calf serum to support a primary antibody response in vitro is determined, in part, by its reduced glutathione content. Cell Immunol. 1980 Aug 1;53(2):325–332. doi: 10.1016/0008-8749(80)90332-9. [DOI] [PubMed] [Google Scholar]
- Hoffman M. K., Schmidt D., Oettgen H. F. Production of antibody to sheep red blood cells by human tonsil cells in vitro. Nature. 1973 Jun 15;243(5407):408–410. doi: 10.1038/243408a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. K. Control of B-cell differentiation by macrophages. Ann N Y Acad Sci. 1979;332:557–563. doi: 10.1111/j.1749-6632.1979.tb47150.x. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp W., Berger R., Posch B. Regulation of pokeweed mitogen-induced human B-cell differentiation: effects of hydrocortisone. Immunopharmacology. 1982 Oct;5(1):1–9. doi: 10.1016/0162-3109(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Knight S. C. Control of lymphocyte stimulation in vitro: "help' and "suppression' in the light of lymphoid population dynamics. J Immunol Methods. 1982;50(1):R51–R63. doi: 10.1016/0022-1759(82)90297-6. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Laemmli U. K. Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell. 1982 May;29(1):171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Luben R. A., Brazeau P., Böhlen P., Guillemin R. Monoclonal antibodies to hypothalamic growth hormone-releasing factor with picomoles of antigen. Science. 1982 Nov 26;218(4575):887–889. doi: 10.1126/science.6813967. [DOI] [PubMed] [Google Scholar]
- Mancino D., Ovary Z. Adjuvant effects of amorphous silica and of aluminium hydroxide on IgE and IgG1 antibody production in different inbred mouse strains. Int Arch Allergy Appl Immunol. 1980;61(3):253–258. doi: 10.1159/000232443. [DOI] [PubMed] [Google Scholar]
- Meretey K., Room G., Maini R. N. Effect of histamine on the mitogenic response of human lymphocytes and its modification by cimetidine and levamisole. Agents Actions. 1981 Apr;11(1-2):84–88. doi: 10.1007/BF01991465. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M., Bayse G. S., Webster R. G. Use of lactoperoxidase catalyzed iodination in immunochemical studies. Immunochemistry. 1971 Mar;8(3):289–297. doi: 10.1016/0019-2791(71)90484-8. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Boswell H. S., Scher I., Singer A. Role of accessory cells in B cell activation. IV. Ia+ accessory cells are required for the in vitro generation of thymic independent type 2 antibody responses to polysaccharide antigens. J Immunol. 1981 Oct;127(4):1345–1347. [PubMed] [Google Scholar]
- Ogden B. E., Hill H. R. Histamine regulates lymphocyte mitogenic responses through activation of specific H1 and H2 histamine receptors. Immunology. 1980 Sep;41(1):107–114. [PMC free article] [PubMed] [Google Scholar]
- Osband M. E., Hamilton D., Shen Y. J., Cohen E., Shlesinger M., Lavin P., Brown A., McCaffrey R. Successful tumour immunotherapy with cimetidine in mice. Lancet. 1981 Mar 21;1(8221):636–638. doi: 10.1016/s0140-6736(81)91554-3. [DOI] [PubMed] [Google Scholar]
- Pardue R. L., Brady R. C., Perry G. W., Dedman J. R. Production of monoclonal antibodies against calmodulin by in vitro immunization of spleen cells. J Cell Biol. 1983 Apr;96(4):1149–1154. doi: 10.1083/jcb.96.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W., Benacerraf B. Immune response in vitro: independence of "activated" lymphoid cells. Science. 1969 Nov 21;166(3908):1002–1004. doi: 10.1126/science.166.3908.1002. [DOI] [PubMed] [Google Scholar]
- Reading C. L. Theory and methods for immunization in culture and monoclonal antibody production. J Immunol Methods. 1982 Sep 30;53(3):261–291. doi: 10.1016/0022-1759(82)90175-2. [DOI] [PubMed] [Google Scholar]
- Sulitzeanu D., Kleinman R., Benezra D., Gery I. Cellular interactions and the secondary response in vitro. Nat New Biol. 1971 Feb 24;229(8):254–255. doi: 10.1038/newbio229254a0. [DOI] [PubMed] [Google Scholar]
- Tarr M. J., Olsen R. G., Jacobs D. L. In vivo and in vitro effects of 1,1-dimethylhydrazine on selected immune functions. Immunopharmacology. 1982 Apr;4(2):139–147. doi: 10.1016/0162-3109(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Van Ness J., Pettijohn D. E. Specific attachment of nuclear-mitotic apparatus protein to metaphase chromosomes and mitotic spindle poles: possible function in nuclear reassembly. J Mol Biol. 1983 Dec 5;171(2):175–205. doi: 10.1016/s0022-2836(83)80352-0. [DOI] [PubMed] [Google Scholar]