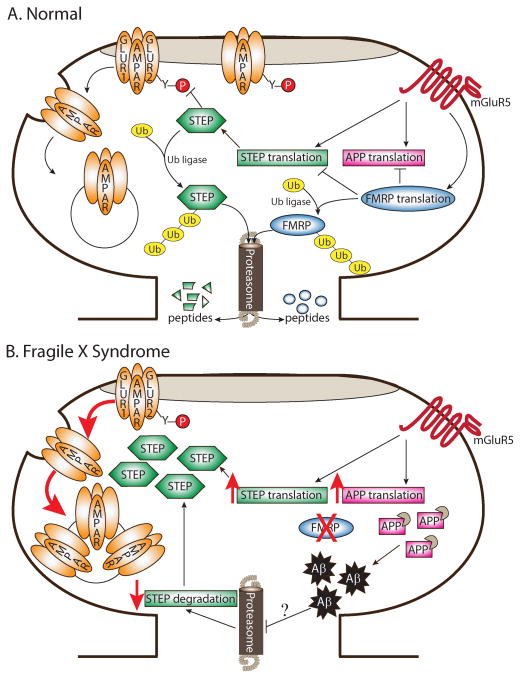
Figure 3. Mechanisms governing STEP protein expression and implications for Fragile X Syndrome.
(A) In normal (or wild-type) neurons, brief stimulation of mGluR5 receptors with DHPG triggers translation of STEP mRNA, as well as translation of other mRNAs including APP and FMRP. STEP dephosphorylates a tyrosine residue on GluR2 and initiates endocytosis of AMPARs following DHPG treatment. FMRP associates with both STEP and APP mRNA and likely acts as a translation suppressor to prevent excessive translation of these mRNAs. Ubiquitination and degradation by the proteasome regulate STEP and FMRP protein levels. Upon stimulation of mGluRs with DHPG, FMRP is rapidly degraded by the proteasome, presumably to permit translation of FMRP targets and allow the expression of LTD. (B) In the absence of FMRP, STEP protein expression might be inappropriately elevated by two parallel pathways. First, without the suppression of STEP mRNA translation by FMRP, the steady-state translation rate of STEP would be upregulated. Similarly, translation of APP is increased in Fmr1 KO mice. Elevated levels of APP provide more targets for β- and γ-secretase-mediated cleavage and result in exacerbated Aβ production in aged Fmr1 KO mice. Given that Aβ inhibits the ubiquitin proteasome system (UPS) in Alzheimer’s disease, it is possible that the UPS is blocked in FXS later in life. Consequently, inhibition of UPS by Aβ could lead to reduced degradation of STEP. Elevated STEP levels in FXS could therefore maintain the persistent internalization of AMPARs and exaggerated mGluR-dependent LTD. Of note, for simplicity, NR2B-containing NMDARs, ERK1/2, and Fyn were removed from this figure; however, it is possible that these proteins would also be more dephosphorylated and inactivated in the presence of elevated STEP levels.