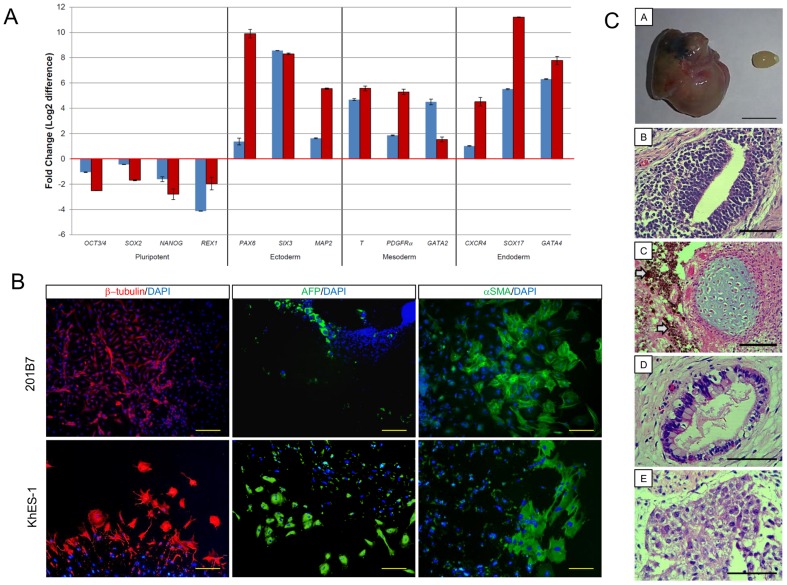
Figure 5. hPSCs maintained differentiation potential after cryopreservation with CP-5E.
(A) hPSCs were cryopreserved with CP-5E. Differentiation of hiPSC (201B7) (blue bar) and hESC (KhES-1) (red bar) was initiated via EB formation after thawing. qRT-PCR was used to assess pluripotency–related genes (OCT4, SOX2, NANOG, and REX1) and 3 germ layer differentiation marker genes (ectodermal [PAX6, SIX3, and MAP2], mesodermal [T, PDGFRα, and GATA2] and endodermal [CXCR4, SOX17, and GATA4]) before and after thawing. Gene expression before and after differentiation were compared by theΔΔCt method. (B) Differentiation of hiPSC (201B7) and hESC (KhES-1) 5 passages after thawing was initiated via EB formation. Molecules related to 3 germ layer differentiation: β-tubulin (ectoderm), α-SMA (mesoderm), or AFP (endoderm) were detected with specific antibodies and visualized with secondary antibodies labeled with Alexa Fluor 488 (green) or Alexa Fluor 546 (red). Nuclei were stained with DAPI. Scale bars: 200 µm. (C) Assessment of post-thaw teratoma formation by hiPSCs. One million hiPSC (201B7) cells cultured for 5 passages after thawing were transplanted under the epidermal space of the left testes of NOG mice; saline was injected in the right testes of the mice as controls. Ten weeks after transplantation, all mice developed teratomas (n = 3). A: photo of a teratoma (left) and control testis (right). Scale Bar: 1 cm. (B–E) Histological analysis of teratoma. Sections were stained with hematoxylin and eosin. B: neural rosette (ectoderm), C: cartilage (mesoderm) and pigmented melanocytes (arrow heads), D: gut-like epithelium (endoderm), E: immature hepatocyte-like cells (endoderm). Scale Bars: 100 µm.