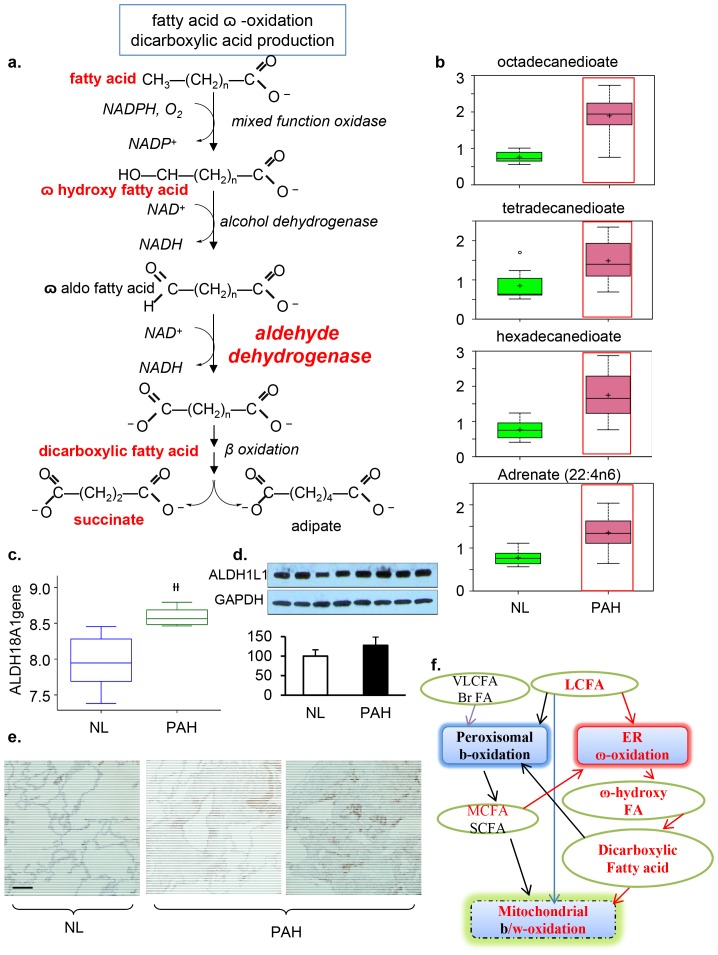
Figure 3. Mitochondrial β–Oxidation Disturbance.
a), fatty acid  –oxidation for dicarboxylic acid production. b) In PAH tissue, there was a significant accumulation of the dicarboxylic fatty acids tetradecanedioate, hexadecanedioate, and octadecanedioate. An accumulation of adrenate (a pro-thrombotic lipid) can cause obstructions that may serve as a biomarker for the disorder. These changes in the lipid profile potentially reflect altered mitochondrial fatty acid oxidation. c), Gene array analysis showed that the gene encoding aldehyde dehydrogenase 18 family, member A1(ALDH18A1) was significantly increased in PAH (p = 1.87e4). d) Western blot analysis of ALDH1L1 expression in normal and PAH lungs. Lung lysate (20 ug per lane) was loaded and immunoblotted with antibody against ALDH1L1 and GAPDH (loading control). Protein expression of ALDH1L1 (40KD) was slightly increased in PAH lungs compared with NL lungs. Densitometric analysis of ALDH was normalized to the intensity of the respective GAPDH band. Data are expressed as mean± SD (n = 4). *P<0.05 versus NL. e) ALDH positive immunostaining in pulmonary vascular smooth muscle cells and endothelial cells in pulmonary vessel of the PAH lung. Representative micrographs of immunostaining of PAH lung sections with anti–ALDH in the pulmonary vascular endothelial cells (bar = 1∶400). f) Dicarboxylic fatty acids are generated when the terminal methyl group of a fatty acid is converted into a carboxyl group and are often indicative of alterations in - ω oxidation. The catabolism of fatty acids typically occurs via β-oxidation in the peroxisomes and/or mitochondria. In contrast, fatty acid
–oxidation for dicarboxylic acid production. b) In PAH tissue, there was a significant accumulation of the dicarboxylic fatty acids tetradecanedioate, hexadecanedioate, and octadecanedioate. An accumulation of adrenate (a pro-thrombotic lipid) can cause obstructions that may serve as a biomarker for the disorder. These changes in the lipid profile potentially reflect altered mitochondrial fatty acid oxidation. c), Gene array analysis showed that the gene encoding aldehyde dehydrogenase 18 family, member A1(ALDH18A1) was significantly increased in PAH (p = 1.87e4). d) Western blot analysis of ALDH1L1 expression in normal and PAH lungs. Lung lysate (20 ug per lane) was loaded and immunoblotted with antibody against ALDH1L1 and GAPDH (loading control). Protein expression of ALDH1L1 (40KD) was slightly increased in PAH lungs compared with NL lungs. Densitometric analysis of ALDH was normalized to the intensity of the respective GAPDH band. Data are expressed as mean± SD (n = 4). *P<0.05 versus NL. e) ALDH positive immunostaining in pulmonary vascular smooth muscle cells and endothelial cells in pulmonary vessel of the PAH lung. Representative micrographs of immunostaining of PAH lung sections with anti–ALDH in the pulmonary vascular endothelial cells (bar = 1∶400). f) Dicarboxylic fatty acids are generated when the terminal methyl group of a fatty acid is converted into a carboxyl group and are often indicative of alterations in - ω oxidation. The catabolism of fatty acids typically occurs via β-oxidation in the peroxisomes and/or mitochondria. In contrast, fatty acid  -oxidation occurs in the smooth endoplasmic reticulum and often serves as a rescue pathway for pathological conditions such as PAH when β-oxidation is disrupted.
-oxidation occurs in the smooth endoplasmic reticulum and often serves as a rescue pathway for pathological conditions such as PAH when β-oxidation is disrupted.