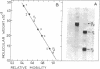
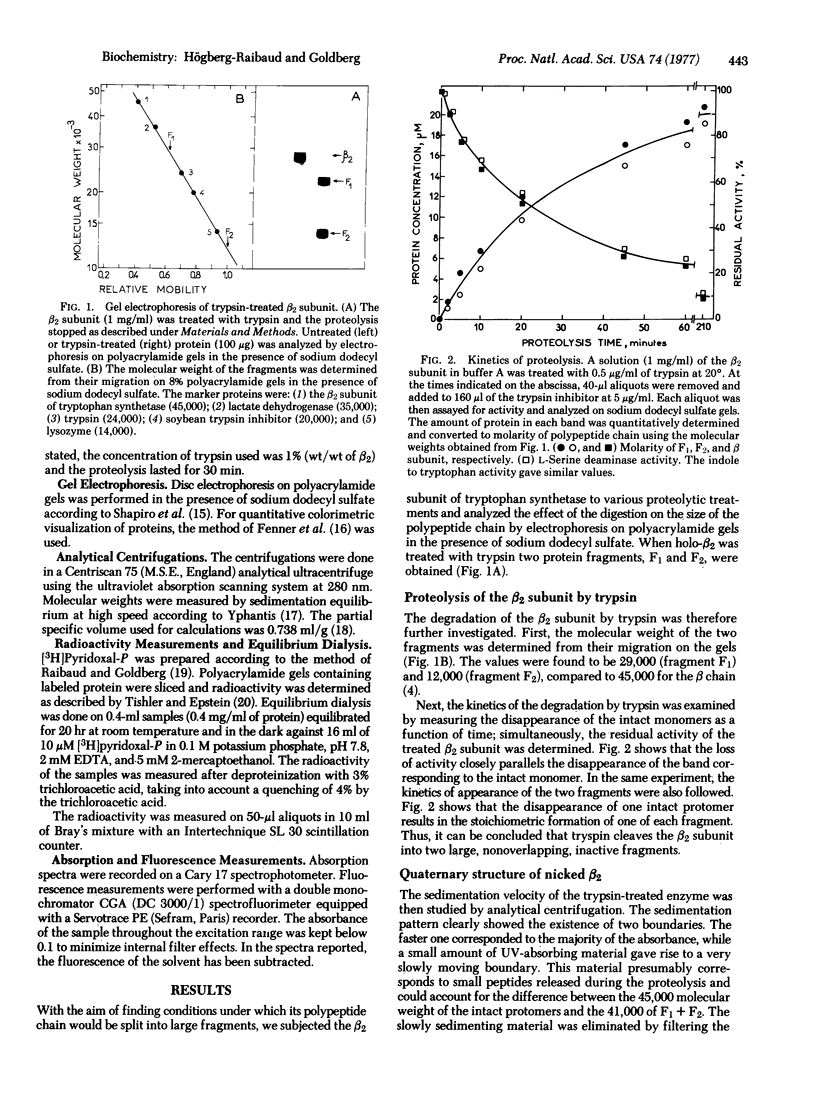
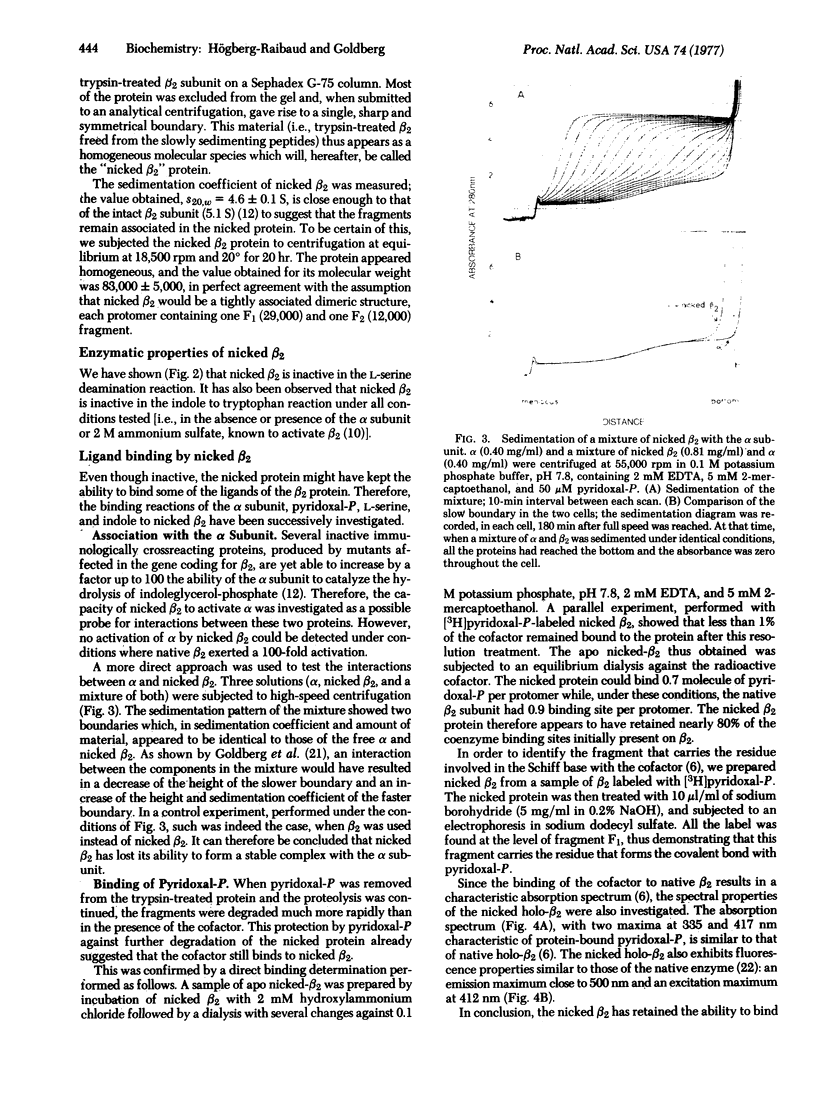
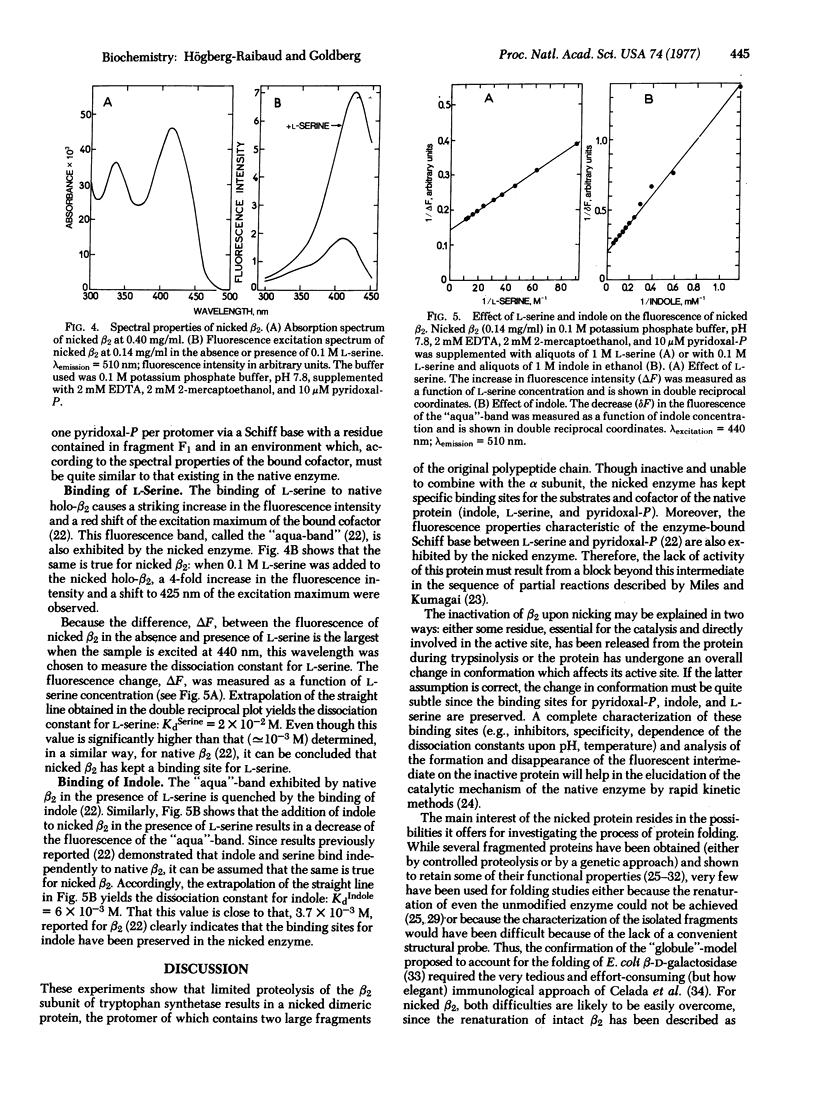
Abstract
Globular proteins often appear to consist of distinct compact "domains," and the assumption is frequently implicitly made that these domains correspond to intermediate structures in the folding process. If this assumption is correct, the polypeptide fragment that builds up a domain should be able to spontaneously fold into its native conformation even when isolated. In an attempt to isolate and study such a fragment, the beta2 subunit of tryptophan synthetase [tryptophan synthase, L-serine hydro-lyase (adding indoleglycerol-phosphate), EC 4.2.1.20] has been subjected to controlled proteolysis. The resulting protein is shown to be a dimer, the protomer of which contains two nonoverlapping polypeptide chains of molecular weights 12,000 and 29,000. Though inactive, the nicked protein is shown to be in a conformation that closely resembles that of the original enzyme, since it still can form an enzyme-bound intermediate of the catalytic reactions. The fluorescence of this intermediate is used to characterize the binding sites for the cofactor (pyridoxal-P) and substrates, which are shown to exist on the nicked protein. The possibility is discussed of using the fragments isolated from the nicked protein to study individual steps of the enzymatic reaction, intracistronic complementation, and the folding process in the normal beta2 subunit.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi O., Miles E. W. A rapid method for preparing crystalline beta 2 subunit of tryptophan synthetase of Escherichia coli in high yield. J Biol Chem. 1974 Sep 10;249(17):5430–5434. [PubMed] [Google Scholar]
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- CRAWFORD I. P., ITO J. SERINE DEAMINATION BY THE B PROTEIN OF ESCHERICHIA COLI TRYPTOPHAN SYNTHETASE. Proc Natl Acad Sci U S A. 1964 Mar;51:390–397. doi: 10.1073/pnas.51.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F., Ullmann A., Monod J. An immunological study of complementary fragments of beta-galactosidase. Biochemistry. 1974 Dec 31;13(27):5543–5547. doi: 10.1021/bi00724a014. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- Faeder E. J., Hammes G. G. Kinetic studies of tryptophan synthetase. Interaction of substrates with the B subunit. Biochemistry. 1970 Oct 13;9(21):4043–4049. doi: 10.1021/bi00823a003. [DOI] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Creighton T. E., Baldwin R. L., Yanofsky C. Subunit structure of the tryptophan synthetase of Escherichia coli. J Mol Biol. 1966 Oct 28;21(1):71–82. doi: 10.1016/0022-2836(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E. Tertiary structure of Escherichia coli beta-D-galactosidase. J Mol Biol. 1969 Dec 28;46(3):441–446. doi: 10.1016/0022-2836(69)90187-9. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., York S., Stryer L. Fluorescence studies of substrate and subunit interactions of the beta-2 protein of Escherichia coli tryptophan synthetase. Biochemistry. 1968 Oct;7(10):3662–3667. doi: 10.1021/bi00850a045. [DOI] [PubMed] [Google Scholar]
- HATANAKA M., WHITE E. A., HORIBATA K., CRAWFORD I. P. A study of the catalytic properties of Escherichia coli tryptophan synthetase, a two-component enzyme. Arch Biochem Biophys. 1962 Jun;97:596–606. doi: 10.1016/0003-9861(62)90129-7. [DOI] [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Hathaway G. M., Kida S., Crawford I. P. Subunit structure of the B component of Escherichia coli tryptophan synthetase. Biochemistry. 1969 Mar;8(3):989–997. doi: 10.1021/bi00831a032. [DOI] [PubMed] [Google Scholar]
- Kida S., Crawford I. P. Complementation in vitro between mutationally altered beta2 subunits of Escherichia coli tryptophan synthetase. J Bacteriol. 1974 May;118(2):551–559. doi: 10.1128/jb.118.2.551-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitt M., Chothia C. Structural patterns in globular proteins. Nature. 1976 Jun 17;261(5561):552–558. doi: 10.1038/261552a0. [DOI] [PubMed] [Google Scholar]
- Miles E. W., Kumagai H. Modification of essential histidyl residues of the beta 2 subunit of tryptophan synthetase by photo-oxidation in the presence of pyridoxal 5'-phosphate and L-serine and by diethylpyrocarbonate. J Biol Chem. 1974 May 10;249(9):2843–2851. [PubMed] [Google Scholar]
- Miles E. W. The B protein of Escherichia coli tryptophan synthetase. I. Effects of sulfhydryl modification on enzymatic activities and subunit interaction. J Biol Chem. 1970 Nov 25;245(22):6016–6025. [PubMed] [Google Scholar]
- Naslin L., Spyridakis A., Labeyrie F. A study of several bonds hypersensitive to proteases in a complex flavohemoenzyme, yeast cytochrome b 2 . Modification of their reactivity with ligand-induced conformational transitions. Eur J Biochem. 1973 Apr;34(2):268–283. doi: 10.1111/j.1432-1033.1973.tb02756.x. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Stryer L. Letter to the editor: Light dissociates enzymatically-cleaved rhodopsin into two different fragments. J Mol Biol. 1975 Jul 5;95(3):477–481. doi: 10.1016/0022-2836(75)90204-1. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Goldberg M. E. A revised method for the tritium labeling of pyridoxal-5'-phosphate. FEBS Lett. 1974 Mar 15;40(1):41–44. doi: 10.1016/0014-5793(74)80889-6. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Goldberg M. E. Characterization of two complementary polypeptide chains obtained by proteolysis of rabbit muscle phosphorylase. Biochemistry. 1973 Dec 4;12(25):5154–5161. doi: 10.1021/bi00749a021. [DOI] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. II. A proteolytic fragment containing the 5' leads to 3' exonuclease function. Restoration of intact enzyme functions from the two proteolytic fragments. J Biol Chem. 1972 Jan 10;247(1):232–240. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Slaby I., Holmgren A. Reconstitution of Escherichia coli thioredoxin from complementing peptide fragments obtained by cleavage at methionine-37 or arginine-73. J Biol Chem. 1975 Feb 25;250(4):1340–1347. [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Véron M., Falcoz-Kelly F., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972 Aug 4;28(4):520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. A., Crawford I. P. Purification and properties of the B component of Escherichia coli tryptophan synthetase. J Biol Chem. 1965 Dec;240(12):4801–4808. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- York S. S. Kinetic spectroscopic studies of substrate and subunit interactions of tryptophan synthetase. Biochemistry. 1972 Jul 4;11(14):2733–2740. doi: 10.1021/bi00764a029. [DOI] [PubMed] [Google Scholar]