Abstract
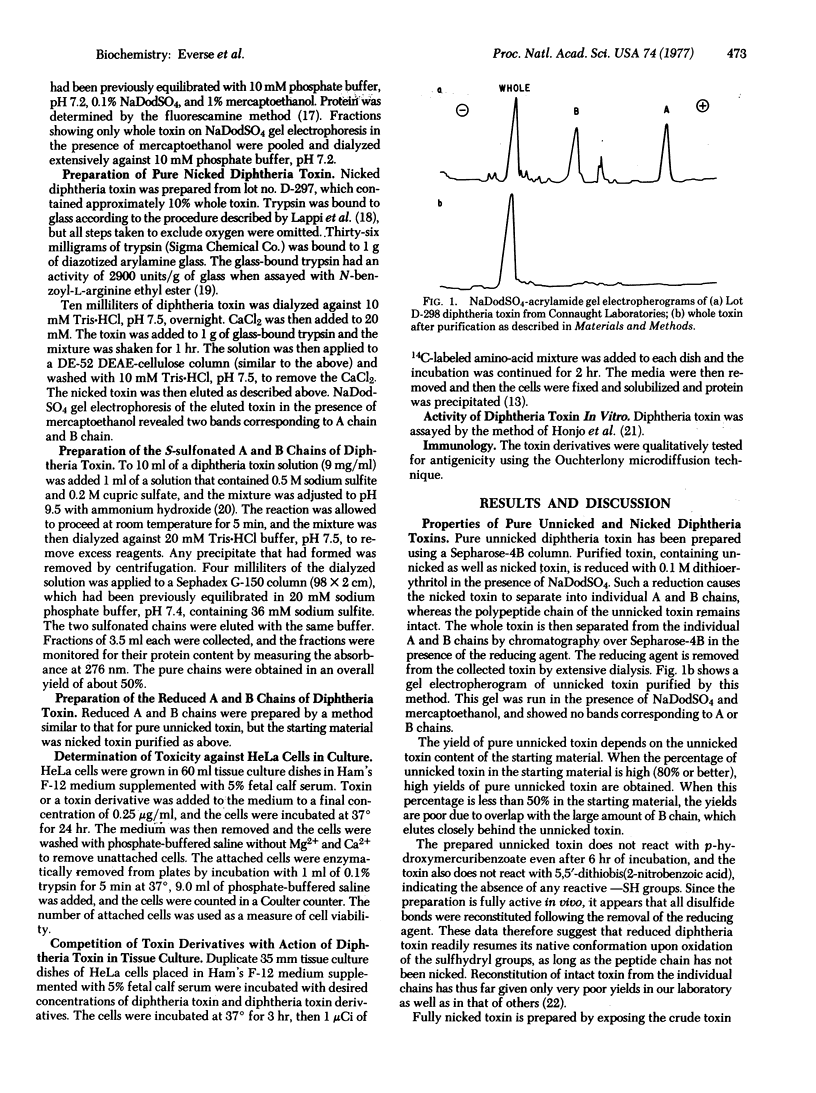
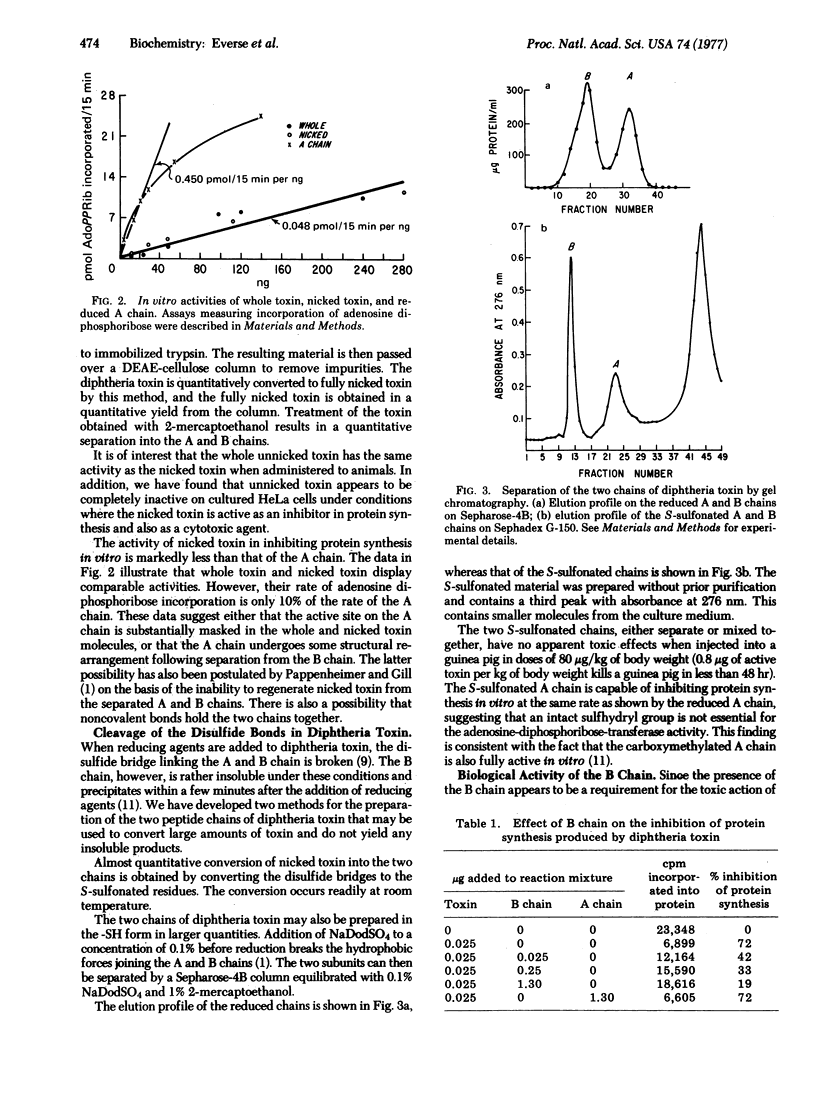
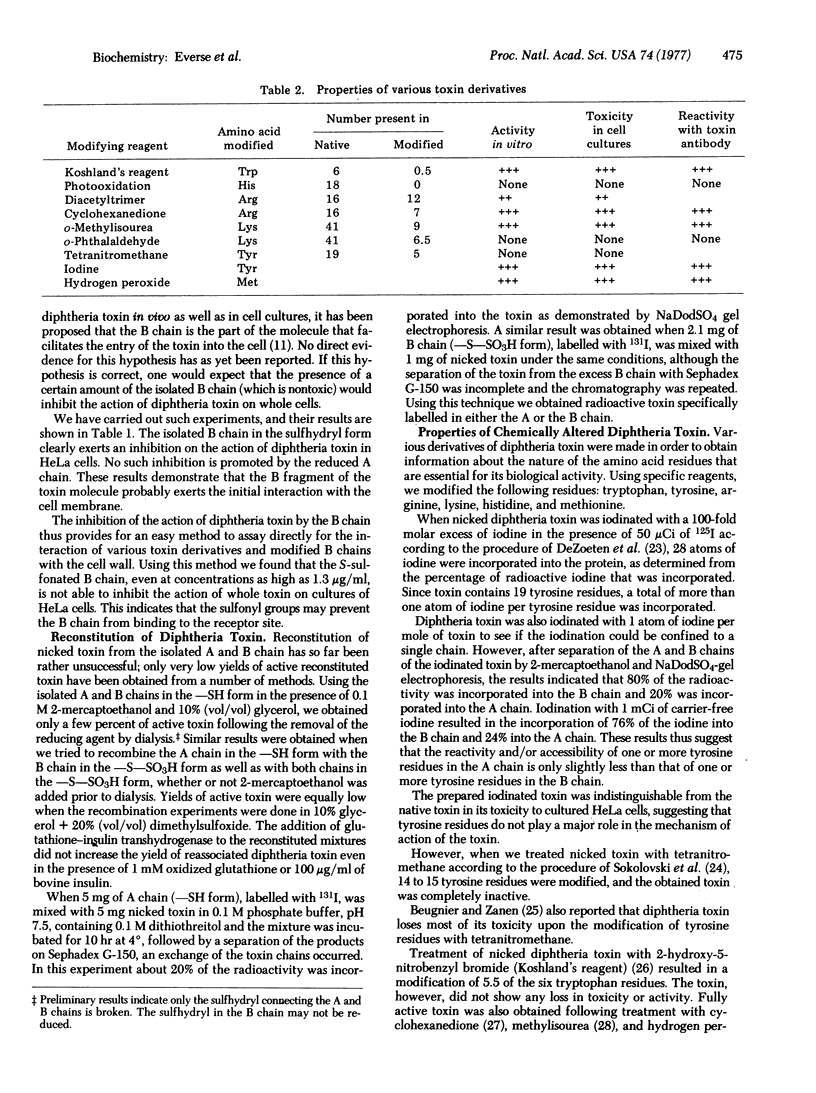
Studies on the structure-function relationship of diphtheria toxin are reported. New methods are described for the preparation of pure intact ("unnicked") toxin and for the preparation of the individual A and B chains. A biological assay method for the B chain is also presented, as well as a method for the labelling of "nicked" (one peptide bond broken) diphtheria toxin with 131I such that the label is confined to only one of the two polypeptide chains. Alterations of diphtheria toxin with specific reagents reveal that modifications of the tryptophan, methionine, and arginine residues did not result in a significant loss in toxicity, whereas treatment of the toxin with omicron-phthalaldehyde or by photooxidation with rose bengal results in a complete loss of the toxic activity. Modification of tyrosine by iodination results in active toxin, whereas modification by tetranitromethane causes a loss in activity. Preliminary results also indicate that the isolated A chain is about an order of magnitude more active in incorporating adenosine diphosphoribose into translocase (elongation factor 2) than whole or nicked toxin is under identical conditions. The observed structural properties are discussed in view of the functional activity of diphtheria toxin in cell-free systems as well as in cell cultures. Evidence is presented indicating that the B chain binds to membranes: it inhibits the action of nicked toxin on HeLa cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barman T. E., Koshland D. E., Jr A colorimetric procedure for the quantitative determination of tryptophan residues in proteins. J Biol Chem. 1967 Dec 25;242(23):5771–5776. [PubMed] [Google Scholar]
- Bellin J. S., Yankus C. A. Influence of dye binding on the sensitized photooxidation of amino acids. Arch Biochem Biophys. 1968 Jan;123(1):18–28. doi: 10.1016/0003-9861(68)90099-4. [DOI] [PubMed] [Google Scholar]
- Beugnier N., Zanen J. Mise en évidence d'une tyrosine dans le site enzymatique de la toxine diphtérique. Arch Int Physiol Biochim. 1973 Sep;81(3):581–581. [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Everse J., Gardner D. A., Kaplan N. O., Galasinski W., Moldave K. The formation of a ternary complex between diphtheria toxin, aminoacyltransferase II, and diphosphopyridine nucleotide. J Biol Chem. 1970 Feb 25;245(4):899–901. [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Goor R. S. New form of diphtheria toxin. Nature. 1968 Mar 16;217(5133):1051–1053. doi: 10.1038/2171051a0. [DOI] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr Studies on the mode of action of diphtheria toxin. 3. Site of toxin action in cell-free extracts. J Exp Med. 1967 Nov 1;126(5):899–912. doi: 10.1084/jem.126.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Kato I., Hayaishi O. Adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis by diphtheria toxin. J Biol Chem. 1971 Jul 10;246(13):4251–4260. [PubMed] [Google Scholar]
- Iglewski B. H., Rittenberg M. B. Selective toxicity of diphtheria toxin for malignant cells. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2707–2710. doi: 10.1073/pnas.71.7.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEE W. A., RICHARDS F. M. The reaction of O-methylisourea with bovine pancreatic ribonuclease. J Biol Chem. 1957 Nov;229(1):489–504. [PubMed] [Google Scholar]
- Lappi D. A., Stolzenbach F. E., Kaplan N. O., Kamen M. D. Immobilization of hydrogenase on glass beads. Biochem Biophys Res Commun. 1976 Apr 19;69(4):878–884. doi: 10.1016/0006-291x(76)90455-1. [DOI] [PubMed] [Google Scholar]
- Michel A., Zanen J., Monier C., Crispeels C., Dirkx J. Partial characterization of diphtheria toxin and its subunits. Biochim Biophys Acta. 1972 Feb 29;257(2):249–256. doi: 10.1016/0005-2795(72)90276-0. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pappenheimer A. M., Jr, Meren R. Lectins from Abrus precatorius and Ricinus communis. II. Hybrid toxins and their interaction with chain-specific antibodies. J Immunol. 1974 Sep;113(3):842–847. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Randall V. On the alleged high sensitivity of mouse Ehrlich-Lettre ascites tumor cells to diphtheria toxin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3149–3152. doi: 10.1073/pnas.72.8.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- SCHACHTER H., DIXON G. H. PREFERENTIAL OXIDATION OF THE METHIONINE RESIDUE NEAR THE ACTIVE SITE OF CHYMOTRYPSIN. J Biol Chem. 1964 Mar;239:813–829. [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Weidekamm E., Wallach D. F., Flückiger R. A new sensitive, rapid fluorescence technique for the determination of proteins in gel electrophoresis and in solution. Anal Biochem. 1973 Jul;54(1):102–114. doi: 10.1016/0003-2697(73)90252-2. [DOI] [PubMed] [Google Scholar]
- Yankeelov J. A., Jr, Mitchell C. D., Crawford T. H. A simple trimerization of 2,3-butanedione yielding a selective reagent for the modification of arginine in proteins. J Am Chem Soc. 1968 Mar 13;90(6):1664–1666. doi: 10.1021/ja01008a056. [DOI] [PubMed] [Google Scholar]


