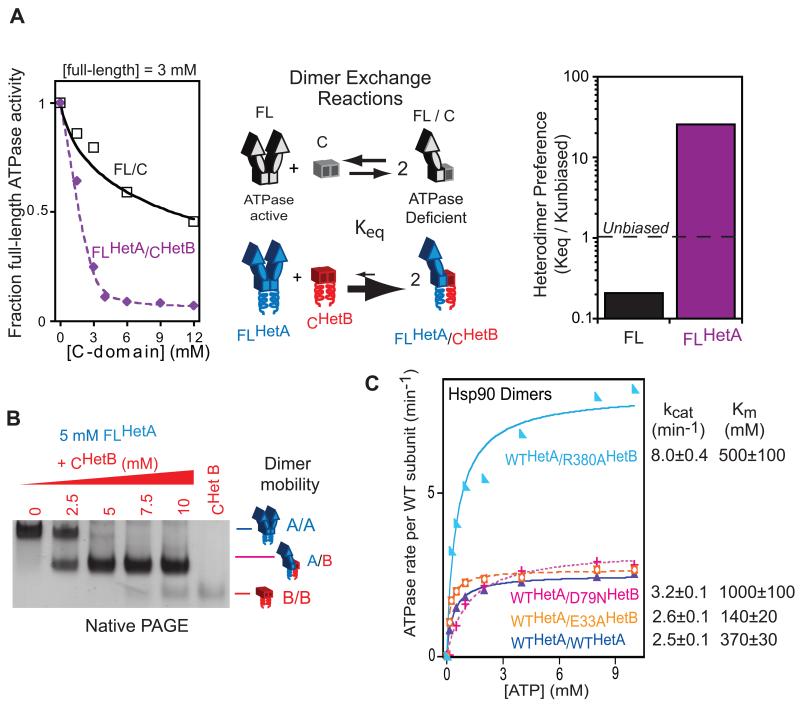
Figure 2.
Designed Hsp90 subunits preferentially assemble as heterodimers in vitro. (A) Dimer exchange reactions monitored based on the ATPase activity from full-length homodimers. Fitting the titrations to an equilibrium model indicates that designed Hsp90 subunits assemble with a roughly 100-fold heterodimer preference relative to wild type controls. (B) Native PAGE confirms the preferential assembly of designed Hsp90 subunits as heterodimers. (C) ATPase activity of Hsp90 heterodimers with ATP binding (D79N) or hydrolysis (E33A, R380A) defective mutations in one subunit. The Michaelis-Menten model was fit to the data to estimate kcat and Km along with errors from the fitting procedure. See also Figure S1.