FIGURE 2. Deletion of the carboxyl-terminal LIM3 and PDZ-binding domains of TRIP6 disrupts its interaction with PTPL1 and abolishes PTPL1-mediated dephosphorylation of TRIP6.
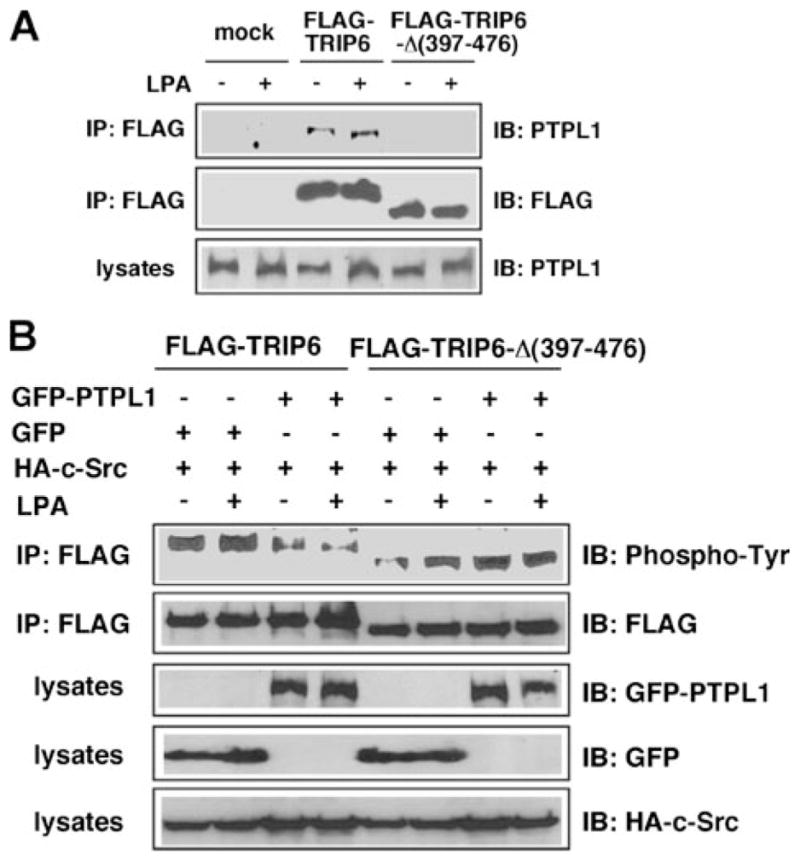
A, a TRIP6-Δ-(397–476) mutant that lacks the LIM3 and PDZ-binding domains cannot bind to PTPL1. HEK 293T cells transiently expressing FLAG-TRIP6 or FLAG-TRIP6-Δ-(397–476) were starved overnight and then stimulated with LPA for 15 min. TRIP6 or TRIP6-Δ-(397–476) was immunoprecipitated with an anti-FLAG M2 monoclonal antibody. After SDS-PAGE, the immunoblot was probed with an anti-PTPL1 antibody. The blot was then stripped and reprobed with an anti-FLAG antibody to detect the immunoprecipitated TRIP6 or TRIP6-Δ-(397–476). The bottom panel shows the expression of endogenous PTPL1 in the whole cell lysates. B, PTPL1 overexpression does not affect tyrosine phosphorylation of TRIP6-Δ-(397–476). FLAG-TRIP6 or FLAG-TRIP6-Δ-(397–476) was expressed in HEK 293T with HA-c-Src, GFP, or GFP-PTPL1 as indicated. After starvation overnight, cells were stimulated with LPA for 15 min. The levels of tyrosine-phosphorylated TRIP6 or TRIP6-Δ-(397–476) were determined as described in panel A. The bottom three panels show the expression of GFP-PTPL1, GFP, and HA-c-Src in the whole cell lysates, respectively. The result shown is representative of three independent experiments.
