Abstract
Background
We examined outcomes and trends in surgery and radiation use for patients with locally advanced esophageal cancer, for whom optimal treatment isn’t clear.
Study design
Trends in surgery and radiation for patients with T1-T3N1M0 squamous cell or adenocarcinoma of the mid or distal esophagus in the SEER database from 1998–2008 were analyzed using generalized linear models including year as predictor; SEER doesn’t record chemotherapy data. Local treatment was unimodal if patients had only surgery or radiation and bimodal if they had both. Five-year cancer specific (CSS) and overall survival (OS) were analyzed using propensity-score adjusted Cox proportional-hazard models.
Results
Overall 5-year survival for the 3,295 patients identified (mean age 65.1 years, SD 11.0) was 18.9% (95%CI: 17.3–20.7). Local treatment was bimodal for 1,274 (38.7%) and unimodal for 2,021 (61.3%) patients; 1,325 (40.2%) had radiation alone and 696 (21.1%) underwent only surgery. The use of bimodal therapy (32.8% to 42.5%, p=0.01) and radiation alone (29.3% to 44.5%, p<0.001) increased significantly from 1998 to 2008. Bimodal therapy predicted improved CSS (HR:0.68, p<0.001) and OS (HR:0.58, p<0.001) compared to unimodal therapy. For the first 7 months (before survival curve crossing), CSS after radiation therapy alone was similar to surgery alone (HR:0.86, p=0.12) while OS was worse for surgery only (HR:0.70, p=0.001). However, worse CSS (HR:1.43, p<0.001) and OS (HR:1.46, p<0.001) after that initial timeframe were found for radiation therapy only.
Conclusions
The use of radiation to treat locally advanced mid and distal esophageal cancers increased from 1998 to 2008. Survival was best when both surgery and radiation were used.
Introduction
Approximately 32% of esophageal cancer patients have regional disease at the time of diagnosis, with a 5-year survival of 10–30%.1, 2 Surgery for locoregional esophageal cancer is utilized in only 30–40% of resectable cases, perhaps because esophagectomy is historically associated with significant morbidity and mortality and disappointing long-term results.3, 4 The role of surgery has also been questioned by two trials that showed treatment with chemoradiation followed by surgery did not improve survival compared to definitive chemoradiation.5, 6 However, important limitations of most esophageal cancer studies that involve surgery are relatively low patient numbers and heterogeneity in the T and N stages of the patients treated. The purpose of this study was to use the Surveillance, Epidemiology, and End Results (SEER) database, which is the largest population-based cancer registry in the United States and contains 17 registries that cover 28% of the US population, to examine local treatment trends in the use of surgery and external beam radiotherapy (EBRT) among patients with locally advanced but potentially resectable T1-3N1M0 esophageal cancer of the mid and distal esophagus to test the hypothesis that combined local therapy was superior to either surgery or EBRT alone in the treatment of these patients. Analysis of a population based cancer registry with advanced statistical methods could provide evidence to support data from prospective randomized trials in this relatively uncommon disease.
Methods
The Duke University Institutional Review Board approved the performance of this secondary SEER database analysis. The following characteristics of patients with esophageal cancer were extracted using SEER*Stat 7.0.5: age (patients age 91 years and older were recoded into the single category of “≥90 years” to meet protected patient health information guidelines), gender, race, ethnicity, marital status, and year of diagnosis. Staging was based on the 6th edition of the AJCC Cancer Staging Manual.7 Tumor-node-metastasis (TNM) stage was manually recoded using available SEER variables for patients in whom TNM stage was not explicitly recorded. When enough tumor information was present in SEER, patients with T3 or T4 tumors were unambiguously recordable while T-stage was classified as T1/2 when T1 and T2 tumors could not be distinguished in the manual recoding. Finally, the following tumor characteristics were collected and grouped: tumor location, histology, grade, primary tumor status (T1, T2, T3, T4, T1/2, unknown), nodal status (N0, N1, unknown), and metastasis status (M0, M1, unknown).
Because SEER did not record specific surgical codes for esophageal cancer prior to 1998, this analysis included only patients from the years 1998 to 2008. Our main goal was to assess local treatment trends and outcomes after surgery only, EBRT only, or treatment with surgery and EBRT, therefore, only patients who underwent one of those treatment options with known sequence of the two treatment components were included in the analysis. From this subset, we included only patients aged 18 years or older having either squamous cell carcinoma (SEER codes 8050–8089) or adenocarcinoma (SEER codes 8140–8389) of the mid or lower esophagus. Because treatment recommendations for cervical esophageal cancer is significantly different than for those of the mid and lower esophagus and the upper thoracic esophagus has a significant overlap with the cervical esophagus, cancers of the cervical and upper thoracic esophagus were not included in this study.8 Finally, only stage T1-3N1M0 patients were included to restrict our analysis to a homogeneous group of esophageal cancer patients with locally advanced, lymph node positive tumors that are still potentially resectable. SEER tumor stage is based on pathological information when surgery was the primary cancer-directed therapy and clinical information if surgery was not performed or if patients had neoadjuvant therapy before esophagectomy.
Patients were grouped as follows: preoperative EBRT and esophagectomy, esophagectomy with postoperative EBRT, esophagectomy only, and EBRT only. Local treatment was further classified into unimodal (EBRT or esophagectomy only) and bimodal therapy (esophagectomy with preoperative or postoperative EBRT). The primary outcome of interest was treatment trends from 1998 to 2008. As secondary outcomes, we investigated cancer-specific (CSS) and overall survival (OS) according to the predefined treatment groups. As the cause of death is included as a distinct variable in SEER, CSS was considered when patients died of esophageal disease. The following three strategies were compared: 1) unimodal versus bimodal, 2) EBRT only versus esophagectomy only, and 3) preoperative versus postoperative EBRT.
Statistical analysis
Demographical and esophageal tumor characteristics were compared using t-test for continuous and chi-square test for categorical variables. Data are shown as means/standard deviations or counts/percentages, where appropriate. The trend over time of the distinct treatment groups was evaluated using logistic regression analysis having year as a continuous predictor in the model. Year was chosen as a continuous variable to best represent the whole time-period of interest with one result while data are shown as Odds ratios (OR) and corresponding 95% confidence intervals (CI). For analyzing OS, patients who were still alive at last follow-up were right censored while for CSS, patients dying from another cause of death than esophageal cancer were also right censored. Overall and cancer specific survival curves using the Kaplan-Meier method to compare unimodal versus bimodal, definitive ERBT only versus surgery only, and preoperative ERBT versus postoperative ERBT were drawn. Statistical comparison was done using the log-rank test. Unadjusted hazard ratios (HR) with corresponding CI were calculated from Cox proportional hazard models for OS for the predefined treatment combinations. Hazard ratios for CSS were estimated using competing-risks regression models according to the method described by Fine and Gray to account for the fact that non-cancer specific death prevents cancer specific death from happening.9
Multivariable adjusted Cox proportional hazard regression analysis were performed including gender, age (≤65 years, >65 years), race, ethnicity, marital status, tumor grade, tumor location, T-stage, histology, and year of diagnosis (5 groups) as a priori defined covariates. Age was stratified as described because that is the age non-disabled patients in the United States become eligible for the Medicare program. The propensity score (PS) was calculated using a multivariable logistic regression model with treatment as the dependent variable and gender, age (≤65 years, >65 years), race, ethnicity, marital status, tumor grade, tumor location, T-stage, histology, and year of diagnosis (5 groups) as independent variables. The Cox proportional hazard models for OS and the competing-risks regression model for CSS were then adjusted using the PS together with the aforementioned a priori defined covariates. Because the survival curves in the subgroup analyses of radiation only versus surgery only crossed and therefore violated the proportionality assumption of the Cox proportional hazard models, we stratified the analysis to the time period before and after the crossing. Main analyses were performed including all patients while subgroup analyses were performed for patients with adenocarcinomas and squamous cell cancers.
We performed two sets of sensitivity analysis. First, we included the number of distinct primary tumors into the propensity score calculation to account for the fact that patients with multiple tumors might have undergone another treatment strategy than patients where the esophageal cancer was first detected. Second we performed a landmark study by left truncating the patients with a survival time below 3-months to acknowledge a potential immortal time bias in regard of receipt of unimodal versus bimodal therapy 10
All statistical calculations were performed using Stata/SE version 11.2 (Stata Corporation, College Station, TX, USA). A significance level (alpha) of 0.05 was chosen for all analyses while all p-values were calculated 2-sided.
Results
SEER analysis identified 3,295 patients with locally advanced (T1-T3N1M0) esophageal cancer who were either locally treated with surgery alone (n=696, 21.1%), EBRT alone (n=1,325, 40.2%), or esophagectomy with EBRT (n=1,274, 38.7%) in the years 1998–2008. Most of the patients had an adenocarcinoma (n=2,221, 67.4%), the minority had a squamous cell carcinoma (n=1,074, 32.6%). During the study period, 855 (26.0%) of the patients underwent preoperative EBRT, 419 (12.7%) postoperative EBRT, 1,325 (40.2%) ERBT only, and 696 (21.1%) esophagectomy only.
Overall, bimodal therapy with surgery and EBRT was performed in 1,274 (38.7%) patients and 2,021 (61.3%) patients underwent unimodal therapy with either surgery or EBRT alone. Table 1 shows the patient and tumor characteristics overall and for the unimodal and bimodal treatment strategy. Compared to patients undergoing bimodality therapy, patients undergoing unimodal therapy were older, had more often mid-esophageal locations and squamous cell histology, were more likely to be female and married, and were less likely to have both stage III disease and a T3 primary tumor.
Table 1.
Comparison of patient and tumor characteristics among unimodal and bimodal therapy as well as for patients with ERBT only and surgery only
| Overall | Unimodal therapy | Bimodal therapy | p-value | ERBT only | Surgery only | p-value | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of patients | 3,295 | 2,021 (61.3) | 1,274 (38.7) | 1,325 (65.6) | 696 (34.4) | ||
|
| |||||||
| Age, (years: mean, standard deviation) | 65.1 (11.0) | 68.0 (10.8) | 60.7 (9.8) | <0.001 | 68.7 (10.7) | 66.6 (10.8) | 0.73 |
| ≤65 | 1,643 (49.9) | 782 (38.7) | 861 (67.6) | <0.001 | 472 (35.6) | 310 (44.5) | <0.001 |
| >65 | 1,652 (50.1) | 1,239 (61.3) | 413 (32.4) | 853 (64.4) | 386 (55.5) | ||
|
| |||||||
| Female | 622 (18.9) | 436 (21.6) | 186 (14.6) | <0.001 | 287 (21.7) | 149 (21.4) | 0.90 |
|
| |||||||
| Race | |||||||
| White | 2,862 (86.9) | 1,705 (84.4) | 1,157 (90.8) | <0.001 | 1,092 (82.4) | 613 (88.1) | 0.001 |
| African-American | 257 (7.8) | 194 (9.6) | 63 (5.0) | 149 (11.3) | 45 (6.5) | ||
| Other/Unknown | 176 (5.3) | 122 (6.0) | 54 (4.2) | 84 (6.3) | 38 (5.5) | ||
|
| |||||||
| Ethnicity | |||||||
| Hispanic | 174 (5.3) | 120 (5.9) | 54 (4.2) | 0.03 | 1,241 (93.7) | 660 (94.8) | 0.29 |
| Non-Hispanic | 3,121 (94.7) | 1,901 (94.1) | 1,220 (95.8) | 84 (6.3) | 36 (5.2) | ||
|
| |||||||
| Marital Status | |||||||
| Married | 2,179 (66.1) | 1,263 (62.5) | 916 (71.9) | <0.001 | 817 (61.7) | 446 (64.1) | 0.29 |
| Other | 1,116 (33.9) | 758 (37.5) | 358 (28.1) | 508 (38.3) | 250 (35.9) | ||
|
| |||||||
| Tumor location | |||||||
| Mid esophagus | 779 (23.6) | 577 (28.6) | 202 (15.9) | <0.001 | 450 (34.0) | 127 (18.3) | <0.001 |
| Lower esophagus | 2,516 (76.4) | 1,444 (71.5) | 1,072 (84.1) | 875 (66.0) | 569 (81.2) | ||
|
| |||||||
| Histology | |||||||
| Adenocarcinoma | 2,221 (67.4) | 1,217 (60.2) | 1,004 (78.8) | <0.001 | 738 (55.7) | 479 (68.8) | <0.001 |
| Squamous cell carcinoma | 1,074 (32.6) | 804 (39.8) | 270 (21.2) | 587 (44.3) | 217 (31.2) | ||
|
| |||||||
| Primary tumor (T) | |||||||
| T1 | 454 (13.8) | 328 (16.2) | 126 (9.9) | <0.001 | 219 (16.5) | 109 (15.7) | <0.001 |
| T2 | 599 (18.2) | 349 (17.3) | 250 (19.6) | 212 (16.0) | 137 (19.7) | ||
| T3 | 2,069 (62.8) | 1,210 (59.9) | 859 (67.4) | 776 (58.6) | 434 (62.4) | ||
| T1/T2 | 173 (5.3) | 134 (6.6) | 39 (3.1) | 118 (8.9) | 16 (2.3) | ||
|
| |||||||
| Tumor stage | |||||||
| IIB | 1,226 (37.2) | 811 (40.1) | 415 (32.6) | <0.001 | 549 (41.4) | 262 (37.6) | 0.10 |
| III | 2,069 (62.8) | 1,210 (59.9) | 859 (67.4) | 776 (58.6) | 434 (62.4) | ||
|
| |||||||
| Tumor grade | |||||||
| G1/2 (well/moderate) | 1,308 (39.7) | 808 (40.0) | 500 (39.3) | 0.11 | 538 (40.6) | 270 (38.8) | <0.001 |
| G3/4 (poor/undifferentiated) | 1,618 (49.1) | 953 (47.2) | 665 (52.2) | 555 (41.9) | 398 (57.2) | ||
| Unknown | 369 (11.2) | 260 (12.9) | 109 (8.6) | 232 (17.5) | 28 (4.0) | ||
|
| |||||||
| Cause of death | |||||||
| Alive | 1,081 (32.8) | 524 (25.9) | 557 (43.7) | <0.001 | 336 (25.4) | 188 (27.0) | 0.69 |
| Esophagus | 1,781 (54.1) | 1,191 (58.9) | 590 (46.3) | 789 (59.6) | 402 (57.8) | ||
| Other cause of death | 433 (13.1) | 306 (15.1) | 127 (10.0) | 200 (15.1) | 106 (15.2) | ||
|
| |||||||
| Number of primary tumors | |||||||
| 1 | 2,727 (82.8) | 1,458 (80.1) | 1,070 (86.8) | <0.001 | 1,055 (79.6) | 566 (81.3) | 0.66 |
| 2 | 467 (14.2) | 294 (16.1) | 143 (11.6) | 216 (16.3) | 104 (14.9) | ||
| ≥3 | 101 (2.9) | 69 (3.8) | 20 (1.6) | 54 (4.1) | 26 (3.7) | ||
Values are counts and % if not otherwise indicated. EBRT: external beam radiotherapy.
From 1998 to 2008, the use of bimodal therapy increased from 32.8%–42.8% (OR per year: 1.03, CI: 1.01–1.06, p=0.01), with a corresponding decrease in unimodal therapy from 67.2%–57.2%. In addition, both EBRT only (29.3%–44.5%; OR per year: 1.05, CI:1.02–1.08, p<0.001) as well as preoperative EBRT (20.7%–32.5%; OR per year:1.08, CI: 1.05–1.11, p<0.001) increased from 1998 to 2008. Conversely, surgery only (37.9%–12.7%; OR per year:0.90, CI: 0.87–0.92, p<0.001) and postoperative EBRT (12.1%–10.3%; OR per year: 0.94, CI: 0.91–0.97, p<0.001) decreased over this time-period. Similar results were found in subgroup analysis for adenocarcinomas while for squamous cell cancer only, the rate of preoperative EBRT and bimodal therapy did not change from 1998 to 2008.
Median follow-up time was 11 months (range:0–130) for patients undergoing unimodal and 16 months (range: 0–125) for patients undergoing bimodal therapy. Overall 5-year survival for all patients was 18.9% (CI:17.3–20.7). Cause of death (esophageal cancer versus other) was similarly distributed among patients undergoing unimodal and bimodal therapy (p=0.13). 5-year CSS and OS for unimodal therapy was 20.7% (CI:18.3–23.3%) and 12.9% (CI:11.1–14.8) while it was 36.4% (CI:32.8–39.9) and 28.8 (CI:25.7–32.0) for bimodal therapy, respectively. In unadjusted survival analysis, the log-rank test favored bimodal therapy over unimodal therapy (Figure 1a) and surgery only over EBRT only (Figure 1b) for both CSS and OS (p<0.001 for all comparisons). No CSS difference was detected for EBRT before versus after surgery (p=0.19) while OS was better in patients undergoing preoperative EBRT (p=0.03) (Figure 1c).
Figure 1.
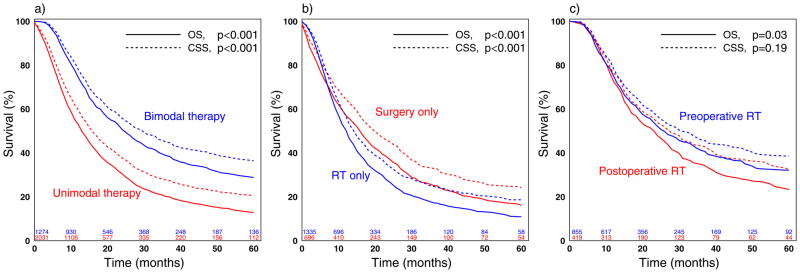
Figure 1a–c: Kaplan-Meier curves: Cancer specific and overall survival comparing a) unimodal versus bimodal therapy, b) definitive external beam radiotherapy (EBRT) only versus surgery only, and c) preoperative EBRT versus postoperative EBRT.
PS-adjusted Cox proportional hazards regression and competing risk analyses for the entire cohort of patients are demonstrated in Table 2. Unimodal therapy (reference group) continued to predict worse CSS (HR:0.67, CI:0.60–0.74, p<0.001) and OS (HR:0.57, CI:0.52–0.62, p<0.001) compared to bimodal therapy. The Kaplan Meier survival curves of EBRT only and surgery alone crossed at 7 months; EBRT alone had a better OS for the first 7 months and similar CSS compared to surgery only, while surgery only showed a better CSS and OS for the following time. When bimodal therapy was used, no statistically significant differences in CSS or OS were observed between preoperative versus postoperative EBRT (p>0.09 for both comparisons). All estimates for patients with adenocarcinomas stayed similar when stratified analysis for adenocarcinoma and for squamous cell carcinoma were performed (Table 3). Bimodal therapy continued to predict better CSS and OS in patients with squamous cell carcinoma. While surgery only for squamous cell carcinoma had similar CSS but worse OS for the first 7 months compared to EBRT only, no such difference was found after the first 7 months. Also, no differences in CSS or OS were seen when preoperative and postoperative EBRT were compared for squamous cell cancer patients.
Table 2.
Cancer specific and overall survival comparing different treatment strategies in all patients
| Cancer specific survival | Overall survival | ||||
|---|---|---|---|---|---|
|
| |||||
| PS-adjusted HR (95% CI) | p-value | PS-adjusted HR (95% CI) | p-value | ||
|
| |||||
| Overall | Unimodal (ref. group) vs. bimodal therapy | 0.67 (0.60–0.74) | <0.001 | 0.57 (0.52–0.62) | <0.001 |
|
| |||||
| Surgery only (ref. group) vs. EBRT only | 1.19 (1.05–1.35) | 0.008 | 1.25 (1.11–1.41) | <0.001 | |
| Surgery only vs. EBRT only, first 7 months | 0.85 (0.70–1.03) | 0.09 | 0.70 (0.58–0.85) | <0.001 | |
| Surgery only vs. EBRT only, 8 to 60 months | 1.33 (1.13–1.56) | <0.001 | 1.47 (1.27–1.71) | <0.001 | |
|
| |||||
| Preoperative (ref. group) vs. postoperative EBRT | 1.03 (0.87–1.22) | 0.74 | 1.14 (0.97–1.33) | 0.10 | |
Estimations for CSS were performed using competing-risks regression model while for OS, cox proportional hazard models were performed. For the calculation of the propensity score (PS), the following covariates were used: gender, age (≤65 years, >65 years), race, ethnicity, marital status, tumor grade, tumor location, tumor T-stage, histology, and year (5 consecutive groups). vs.=versus, EBRT = External beam radiotherapy, ref.=reference, HR=Hazard ratio, CI=95% confidence interval
Table 3.
Cancer specific and overall survival comparing different treatment strategies stratified by tumor histology.
| Cancer specific survival | Overall survival | ||||
|---|---|---|---|---|---|
|
| |||||
| PS-adjusted HR (95% CI) | p-value | PS-adjusted HR (95% CI) | p-value | ||
|
| |||||
| Adenocarcinoma | Unimodal (ref. group) vs. bimodal therapy | 0.68 (0.60–0.76) | <0.001 | 0.56 (0.50–0.62) | <0.001 |
|
| |||||
| Surgery only (ref. group) vs. EBRT only | 1.21 (1.03–1.42) | 0.02 | 1.34 (1.15–1.55) | <0.001 | |
| Surgery only vs. EBRT only,.first 7 months | 0.81 (0.64–1.04) | 0.10 | 0.71 (0.56–0.90) | 0.005 | |
| Surgery only vs. EBRT only, 8 to 60 months | 1.40 (1.14–1.71) | 0.001 | 1.64 (1.36–1.97) | <0.001 | |
|
| |||||
| Preoperative (ref. group) vs. postoperative EBRT | 0.92 (0.76–1.12) | 0.42 | 1.05 (0.88–1.25) | 0.58 | |
|
| |||||
| Squamous cell cancer | Unimodal (ref. group) vs. bimodal therapy | 0.64 (0.53–0.79) | <0.001 | 0.59 (0.49–0.71) | <0.001 |
|
| |||||
| Surgery only (ref. group) vs. EBRT only | 1.13 (0.91–1.40) | 0.27 | 1.09 (0.90–1.33) | 0.38 | |
| Surgery only vs. EBRT only,.first 7 months | 0.86 (0.62–1.19) | 0.36 | 0.68 (0.49–0.95) | 0.02 | |
| Surgery only vs. EBRT only, 8 to 60 months | 1.18 (0.90–1.54) | 0.24 | 1.19 (0.93–1.52) | 0.18 | |
|
| |||||
| Preoperative (ref. group) vs. postoperative EBRT | 1.38 (0.93–2.04) | 0.11 | 1.38 (0.97–1.95) | 0.08 | |
Estimations for CSS were performed using competing-risks regression model while for OS, cox proportional hazard models were performed. For the calculation of the propensity score (PS), the following covariates were used: gender, age (≤65 years, >65 years), race, ethnicity, marital status, tumor grade, tumor location, tumor T-stage, histology, and year (5 consecutive groups). vs.=versus, EBRT = External beam radiotherapy, ref.=reference, HR=Hazard ratio, CI=95% confidence interval
Sensitivity Analysis
First: all main analysis estimates remained stable without change in any significance levels when the number of distinct primary tumors was included in the PS calculation. Second: the recalculated estimates for the main analyses remained stable even after left truncating patients with a survival time of less than 3 months (data not shown).
Discussion
Patients with esophageal cancer and lymph node involvement who are treated with surgery alone have a five-year survival of 15–40%.11 Many studies involving various combinations of surgery, chemotherapy, and EBRT have been conducted in efforts to improve these relatively poor long-term results. The National Comprehensive Cancer Network (NCCN) guidelines reflect the lack of available definitive data on the optimal local treatment for esophageal cancer without distant metastases.8 These guidelines allow for the entire spectrum of possible treatment for tumors that at least invade the muscularis propria, including any combination of esophagectomy and chemoradiation. In this study with analysis limited to patients with potentially surgically resectable esophageal cancer of the mid and distal esophagus in the presence of nodal disease, we have identified that induction EBRT followed by surgery and definitive EBRT are being used with increasing frequency. In addition, we have identified that combined therapy with surgery and EBRT is associated with superior outcomes compared to either surgery or EBRT alone.
The results of this study are consistent with other studies that have shown that EBRT is an important determinant of survival in patients with node positive disease treated with surgical resection.12, 13 Although randomized trials have shown conflicting results when comparing multi-modality therapy with surgery alone, several meta-analyses have shown a benefit to induction therapy followed by surgical resection.14–18 Limitations of those previous studies were that they included a heterogeneous group of T and N stages and in some cases, gastric cancer. An advantage of our current study is that a large number of this particular subset of patients could be identified for analysis.
The potential benefits of multi-modality therapy in previous studies may have been offset by increased treatment mortality when surgery was included, especially considering that these studies contained relatively small numbers of patients.5, 6, 19 Indeed, a critical factor when considering surgical resection for esophageal cancer is the potential perioperative morbidity and mortality. Historically high mortality rates associated with esophagectomy likely have limited the use of surgical resection.3, 4 Although recent data from high-volume centers have shown low mortality rates of 1 to 3.5%, studies involving population-based databases or multi-center trials show that esophageal resection continues to have relatively high perioperative mortality rates of 8.8 to 14%.20–24 In this study, perioperative mortality appeared to be significant in that patients treated with surgery appeared to have worse outcomes in the first seven months after diagnosis compared to those treated without surgery. However, despite the initially worse observed results when surgery was involved, overall patients treated with surgery had better long-term outcomes. These findings underscore the importance of limiting surgical morbidity so that both overall outcomes can be optimized and that surgery can be applied in more cases.
In this study, the timing of EBRT when combined with surgery did not show any impact on survival. There are theoretical advantages to both preoperative and postoperative EBRT. Postoperative EBRT may reduce the incidence of local recurrence in patients who have residual tumor after resection, though it is not clear if postoperative EBRT is beneficial in the absence of residual disease.1, 25, 26 The potential advantages of preoperative EBRT include tumor downstaging that may improve resectability, improving tolerance as compared to postoperative treatment, avoiding potential EBRT damage to the conduit used for esophageal replacement, and allowing time for the detection of distant metastatic disease that therefore avoids morbidity in patients who would not be expected to derive benefits from aggressive surgical resection. However, randomized controlled studies are needed to better define the optimal timing of therapy when combining surgery and EBRT.
The SEER dataset does have inherent limitations. First, data regarding chemotherapy administration is missing. The impact of this limitation on our finding that combined therapy is better than unimodal therapy is not clear. Many if not most of the patients in our study population who received EBRT were likely to also have had chemotherapy as early randomized trials showed that definitive chemoradiation was superior to EBRT alone.26–29 Patients undergoing surgery without EBRT may have been less likely to have received chemotherapy, which could have negatively biased the results seen with surgery as unimodal therapy. Second, data regarding patient co-morbidities and performance status were missing. These factors are potentially important in predicting both survival as well as treatment, particularly surgery, and would have strengthened the propensity score calculation if they had been available. Third, information on complete/incomplete resections as well as on response to EBRT are not collected in SEER which therefore can not be included in the analysis. The restriction of only including T1-T3 tumors in the analysis likely limited the number of patients who had R2 resections with gross residual disease, but some patients may have had R1 resections with positive microscopic margins. Fourth, our analysis is based on staging information according to the UICC 6th edition and not to the newer UICC 7th edition. However, the only staging changes that would affect our patient population are the more detailed stratifications of nodal status in UICC 7th edition. Although this stratification does improve the survival prediction, this staging change has not yet impacted on treatment recommendations that could impact our analysis. Fifth, despite accumulating a relatively large homogenous group of patients with the SEER database, our analysis may still be underpowered to detect small differences in outcomes, particularly between patients treated with induction or adjuvant radiation therapy. Unfortunately, considering the relative uncommon nature of esophageal cancer, accumulating a larger number of patients via another dataset or via a prospective study is unlikely. Finally, the overall follow-up period for the patients in the study is relatively short.
An additional limitation is that the differences in outcomes for the various therapies may have been biased by the way staging is recorded by SEER. The recorded SEER stage is based on pathological examination in patients who underwent esophageal resection as their first treatment, while the clinical stage prior to treatment is the recorded SEER stage for patients who underwent preoperative EBRT or EBRT only. Considering that clinical staging is not as reliable as pathological staging, patients who did not have surgery as their initial treatment may have been under-staged or over-staged, which could have either negatively or positively biased the results seen for patients who had surgery as their first treatment. The recorded clinical stage is further potentially biased by the method of staging, considering that endoscopic ultrasound, particularly when combined with fine-needle aspiration, is much more accurate than CT scans for nodal staging.30 Unfortunately, the specific staging modalities used are not recorded in SEER, and the impact of staging bias cannot really be estimated.
However, the strength of using SEER for this investigation is the power to assess almost 3,300 patients with locally advanced mid and distal esophageal cancer, which allows both subset analysis and major generalization of the results as SEER represents about 28% of the United States population. Any clinical trial is extremely unlikely to ever be able to assemble a similar number of patients. The results of this population-based analysis show that treatment has changed over time such that radiation and combined modality therapy is increasingly used, but that treatment is still somewhat variable. This treatment variability is not necessarily surprising, given that guidelines allow a wide variety of treatment possibilities.8 However, the results show improved outcomes with combined modality treatment, which is consistent with data from recently published randomized trials.18 Considering that patient treatment in SEER reflects clinical practice outside of the realm of potentially selective controlled clinical trials, these results show that the benefits of combined modality treatment seen in randomized trial are also observed in a population-based setting and further support the treatment of locally advanced esophageal cancer with combined EBRT and surgery.
Acknowledgments
Research support:
This work was in part supported by grant PBBEP3-131567 from the Swiss National Science Foundation (MW) and the NIH funded Cardiothoracic Surgical Trials Network (MFB).
Footnotes
Presentation:
Part of this work was presented as an oral presentation at the Annual Congress of the Swiss Society for Surgery (Davos, June 2012).
The authors have no other potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 3.Paulson EC, Ra J, Armstrong K, Wirtalla C, Spitz F, Kelz RR. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143:1198–203. doi: 10.1001/archsurg.143.12.1198. discussion 203. [DOI] [PubMed] [Google Scholar]
- 4.Dubecz A, Sepesi B, Salvador R, Polomsky M, Watson TJ, Raymond DP, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg. 2010;211:754–61. doi: 10.1016/j.jamcollsurg.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 6.Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–7. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL. AJCC cancer staging manual. 6. New York: Springer-Verlag; 2002. American Joint Committee on Cancer and American Cancer Society; p. xiv.p. 421. [Google Scholar]
- 8.Ajani JA, Barthel JS, Bentrem DJ, D’Amico TA, Das P, Denlinger CS, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–87. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 9.Fine JP, Gray RJ. A proportional hazards model the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 10.Park HS, Gross CP, Makarov DV, Yu JB. Immortal Time Bias: A Frequently Unrecognized Threat to Validity in the Evaluation of Postoperative Radiotherapy. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 12.Schwer AL, Ballonoff A, McCammon R, Rusthoven K, D’Agostino RB, Jr, Schefter TE. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: a surveillance, epidemiology, and end-results study. Int J Radiat Oncol Biol Phys. 2009;73:449–55. doi: 10.1016/j.ijrobp.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Solomon N, Zhuge Y, Cheung M, Franceschi D, Koniaris LG. The roles of neoadjuvant radiotherapy and lymphadenectomy in the treatment of esophageal adenocarcinoma. Ann Surg Oncol. 2010;17:791–803. doi: 10.1245/s10434-009-0819-4. [DOI] [PubMed] [Google Scholar]
- 14.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 15.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–68. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 16.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 18.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 19.Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–7. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 20.Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–22. doi: 10.1016/s0003-4975(02)04368-0. discussion 22. [DOI] [PubMed] [Google Scholar]
- 21.Rentz J, Bull D, Harpole D, Bailey S, Neumayer L, Pappas T, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–20. doi: 10.1067/mtc.2003.315. [DOI] [PubMed] [Google Scholar]
- 22.Ra J, Paulson EC, Kucharczuk J, Armstrong K, Wirtalla C, Rapaport-Kelz R, et al. Postoperative mortality after esophagectomy for cancer: development of a preoperative risk prediction model. Ann Surg Oncol. 2008;15:1577–84. doi: 10.1245/s10434-008-9867-4. [DOI] [PubMed] [Google Scholar]
- 23.Berry MF, Atkins BZ, Tong BC, Harpole DH, D’Amico TA, Onaitis MW. A comprehensive evaluation for aspiration after esophagectomy reduces the incidence of postoperative pneumonia. J Thorac Cardiovasc Surg. 2010;140:1266–71. doi: 10.1016/j.jtcvs.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–72. doi: 10.1097/SLA.0b013e31814697f2. discussion 72–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fok M, Sham JS, Choy D, Cheng SW, Wong J. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery. 1993;113:138–47. [PubMed] [Google Scholar]
- 26.Teniere P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet. 1991;173:123–30. [PubMed] [Google Scholar]
- 27.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. Jama. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 28.Arnott SJ, Duncan W, Kerr GR, Walbaum PR, Cameron E, Jack WJ, et al. Low dose preoperative radiotherapy for carcinoma of the oesophagus: results of a randomized clinical trial. Radiother Oncol. 1992;24:108–13. doi: 10.1016/0167-8140(92)90287-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Gu XZ, Yin WB, Huang GJ, Wang LJ, Zhang DW. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: report on 206 patients. Int J Radiat Oncol Biol Phys. 1989;16:325–7. [PubMed] [Google Scholar]
- 30.Vazquez-Sequeiros E, Wiersema MJ, Clain JE, Norton ID, Levy MJ, Romero Y, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626–35. doi: 10.1053/j.gastro.2003.08.036. [DOI] [PubMed] [Google Scholar]


