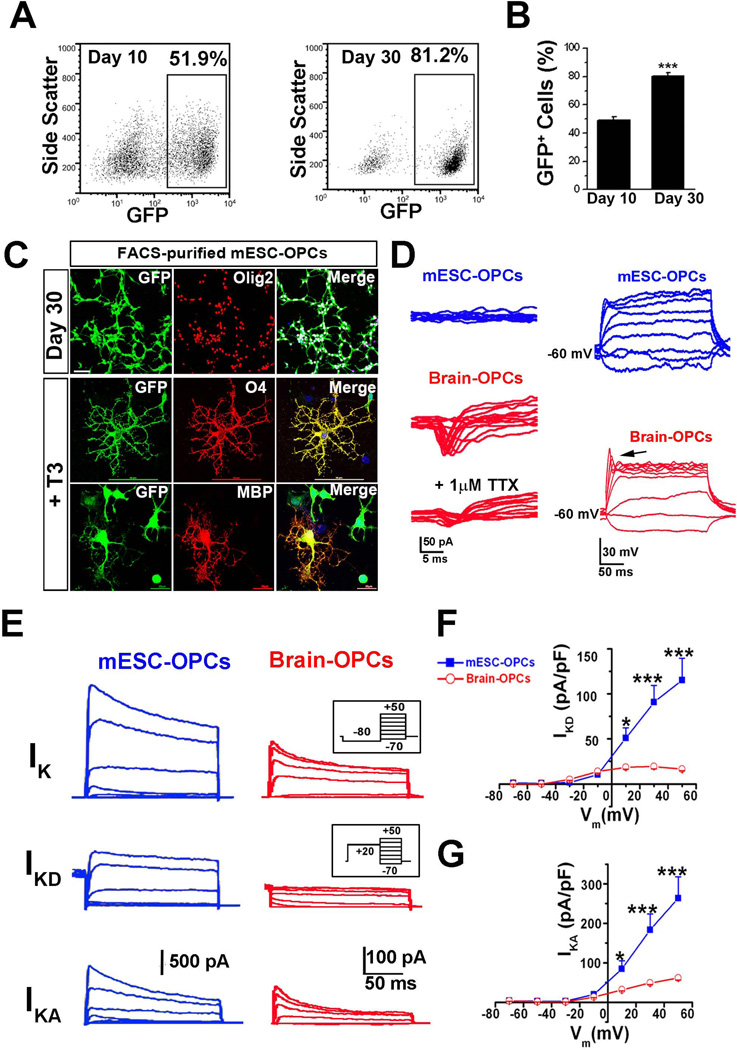
Figure 2. Comparison of electrophysiological properties between GFP+ mESC-OPCs and cultured brain OPCs.
(A and B) Representative FACS analysis (A) and quantitative analysis (B) showing that the percentage of Olig2+/GFP+ cells dramatically increased from day 10 to day 30 (n = 4). (C) Representative images showing that the FACS-purified day 30 mESC-OPCs were co-labeled by GFP and Olig2, and matured into O4+ and MBP+ oligodendrocytes in the presence of T3. The scale bars for Olig2 and O4 staining represent 50 µm. The scale bar for MBP staining represents 20 µm. Blue, DAPI-stained nuclei. (D) Right panels, representative tracing showing that no INa was recorded in the mESC-derived OPCs in the presence of Cs+ internal solution (upper panel), while the TTX-sensitive INa was present in cultured brain OPCs (middle and bottom panels). The INa was elicited by a series of depolarizing voltage steps (from −70 to −50 mV) after a pre-pulse to −100 mV for 100 ms. Similar results were observed from 10 other cells for each group. Left panels, representative tracing showing that no spikes were recorded in the mESC-OPCs even when the cell is depolarized to 0 mV (upper panel), while slight spikes were observed in cultured brain OPCs (indicated by the arrow in the lower panel). Current injection: 300 ms, 10 pA steps from −20 to 60 pA. (E) Superimposed current recording in the presence of TTX from a GFP+ mESC-OPC and a typical cultured brain OPC under voltage clamp at different voltages (200 ms, 20 mV steps from −70 mV to −50 mV) preceded by a pre-pulse conditioning potential of −80 mV, 300 ms (inset, upper panels) or +20 mV, 300 ms (inset, middle panels). With a pre-pulse to −80 mV, overall potassium currents (upper panels) including IKA and sustained outward current IKD was recorded, whereas only IKD (middle panels) could be recorded with a pre-pulse to +20 mV that inactivates IKA component. The traces in bottom panels were of the IKA component and obtained by subtracting the middle panels from the upper panels. (F and G) I-V relationships of the IKD (F) and IKA (G) recorded from mESC-OPCs and cultured brain OPCs (n = 7 for each type of cells). * p < 0.05 and *** p < 0.001. Data are presented as mean ± s.e.m.. Abbreviations: mESCs, mouse embryonic stem cells; FACS, fluorescence-activated Cell Sorting; T3, 3,3´5´-triido-L-thyronine; OPCs, oligodendrocyte progenitor cells; TTX, tetrodotoxin; INa, voltage-gated sodium channel-mediated current; IKA, inactivating A-type potassium current; IKD, delayed rectifier potassium current.