Abstract
High visual acuity allows parallel processing of distant environmental features, but only when photons are abundant enough. Diurnal tiger beetles (Carabidae: Cicindelinae) have acute vision for insects and visually pursue prey in open, flat habitats. Their fast running speed causes motion blur that degrades visual contrast, forces stop-and-go pursuit and potentially impairs obstacle detection. We demonstrate here that vision is insufficient for obstacle detection during running, and show instead that antennal touch is both necessary and sufficient for obstacle detection. While running, tiger beetle vision appears to be photon-limited in a way reminiscent of animals in low-light habitats. Such animals often acquire wide-field spatial information through mechanosensation mediated by longer, more mobile appendages. We show that a nocturnal tiger beetle species waves its antennae in elliptical patterns typical of poorly sighted insects. While antennae of diurnal species are also used for mechanosensation, they are rigidly held forward with the tips close to the substrate. This enables timely detection of path obstructions followed by an increase in body pitch to avoid collision. Our results demonstrate adaptive mechanosensory augmentation of blurred visual information during fast locomotion, and suggest that future studies may reveal non-visual sensory compensation in other fast-moving animals.
Keywords: antenna, vision, insect locomotion, motion blur
1. Introduction
Obtaining and acting on long-range information about prey, mates, predators and spatial structure of the environment is one of the most universal behaviours [1]. Although a variety of sensory modalities may be used, such information is best acquired by vision [2]. This modality can enable acute pattern recognition and long-range extraction of depth cues that allows planning of adaptive actions. Furthermore, visual systems typically process wide-field spatial information in parallel. Thus, for creatures active where photons are abundant, vision is the modality that has been predominantly selected to gather spatial information [3]. When photons become scarce, however, animals must compensate. Some animals living in low-photon habitats, such as crepuscularly active bees [4], still rely on vision and have evolved morphological and physiological specializations to increase capture of the photons available at dusk and dawn. Animals that live in darker habitats use another sensory modality and often gather spatial information by passive audition [5] or by actively probing their environment using self-generated energy, such as echolocation or electrolocation [6]. When terrestrial animals make a transition to such habitats, they frequently evolve reduced visual abilities and increased mechanosensory abilities [7,8]. Vibrissae [9] and antennae [10] are moved around actively to acquire information and often become longer. Cockroaches, for example, are active in low-light conditions and rely on antennal cues, not on vision, in obstacle negotiation [11,12]. Their antennae sweep wide horizontal and vertical arcs and are in motion while the cockroach moves through open spaces [12,13]. This active mechanosensory strategy is taken to an extreme in animals such as stick insects [14] and whipspiders (Amblypygi) [15], which use their long antenniform legs to map three-dimensional space.
The tiger beetles (Carabidae: Cicindelinae) are a large group of predatory beetles that includes both nocturnal and diurnal species. Nocturnal tiger beetles are generalist, cursorial predators with small eyes that have relatively low spatial acuity [16]. As such, they are likely to use antennal movements similar to other nocturnal or poorly sighted insects. By contrast, diurnal tiger beetle species have some of the best vision among insects. Members of the genus Cicindela have large eyes [16] with a horizontal acute zone in which interommatidial angles are less than 1° [17,18]. They are generalist predators that run their prey down under visual guidance [19] in relatively open, flat habitats [20]. Diurnal tiger beetles are extremely fast runners, with some species exceeding 2.4 m s−1 or 120 body lengths per second [21]. This speed comes at the cost of significant visual motion blur that reduces the perceived contrast of the beetle's prey so much that the target is ‘lost’, and the beetle must stop during pursuit to relocalize the prey [19]. After a few tens of milliseconds, depending upon prey movement, the target is reacquired, and the beetle accelerates again in its direction. After three or four iterations of their characteristic stop–go pursuit, the prey is captured.
Based on the importance of vision in the behaviour of diurnal tiger beetles, one would expect that vision is their primary modality for the acquisition of spatial information. However, the susceptibility to visual motion blur that affects target tracking during locomotion might also be predicted to impair negotiation of surface topography and obstacles. If beetles are functionally blind while running, then how do they avoid collisions and tripping? This question led us to examine the role of their antennae during fast locomotion, and test whether antennal mechanosensation can compensate for the decreased capacity of the visual system to acquire spatial information. We show here that the antennae are sufficient and, indeed, necessary for acquiring such spatial information about the beetle's environment during running. The eyes play little role.
2. Material and methods
(a). Beetle husbandry
Hairy-necked tiger beetles, Cicindela hirticollis (Carabidae: Cicindelinae), were collected in an abandoned quarry near Cortland (Cortland County, New York, NY, USA). Greater night-stalking tiger beetles, Omus dejeanii, were collected in Oregon (USA) and supplied by Peter Clausen (www.bugsincyberspace.com). Beetles were housed individually in transparent plastic containers (13 × 18 × 7 cm) in the laboratory at room temperature. The floor of the container was covered with a mixture of soil and sand that was kept slightly damp. Beetles were fed, twice a week, with five to eight mutant strain (AABYS) houseflies (Musca domestica) that can barely fly. Each C. hirticollis beetle had the left hindwing clipped to prevent flight and escape from the containers or test arena, which previous work [19] has shown to have no effect on locomotory behaviour. This was not necessary for O. dejeanii as their elytrae are fused.
(b). Experimental set-up
Experiments with C. hirticollis were performed in a rectangular glass arena (30 cm long × 10 cm wide × 6.5 cm high). Beetles were filmed at 400 frames per second with a high-speed camera (Phantom v. 5.0, Vision Research Inc., Wayne, NJ, USA) fitted with a 50 mm Nikon prime lens. Two mirrors were arranged at 45° angles, so lateral and dorsal views of the beetle were visible in the same video frame. A small aperture was chosen for maximal depth of field, in order to keep both views in focus. All sides of the arena except for one long side were made opaque by taping sheets of tracing paper to the glass. Obstacles were made from stacks of standard glass microscope slides that were cut to the same width as the arena. Low, medium and high obstacles (two, three or four slides, each 1 mm high) were glued together with Scotch super glue (3M). ‘Low-contrast’ obstacles were untreated glass, with a strip of yellow masking tape on top (same as the arena floor). ‘High-contrast’ obstacles were spray-painted with matte black paint. The arena was illuminated with two 45 W compact fluorescent bulbs. Temperature in the arena was kept between 25°C and 30°C. Beetles were acclimatized to the arena for 10 min before starting the protocol (see below).
(c). Protocols
First, runs from unimpaired beetles were filmed in four conditions: no obstacle, low (2 mm), medium (3 mm) and high (4 mm) low-contrast obstacles (N = 20 beetles, n = 4 obstacle conditions, n = 10 runs per obstacle height (incl. zero): 800 runs). Order of obstacles was randomized. The 20 beetles were then assigned to four equal-sized groups for further experimentation. Group 1 consisted of unimpaired beetles with high-contrast obstacles, group 2 beetles were blinded by painting over their eyes with red enamel paint. In groups 3 and 4, antennae were clipped distal of the scape; group 3 then ran over low-contrast obstacles and group 4 over high-contrast obstacles. Groups 2–4 were not tested for at least 48 h after treatment. Beetles mostly ran back and forth in the arena without external influence, but occasionally, runs were elicited by moving a paint brush behind the beetle. Each group member performed a total of 40 runs, with 2 min pauses between runs, and 30 min pauses when changing obstacle condition.
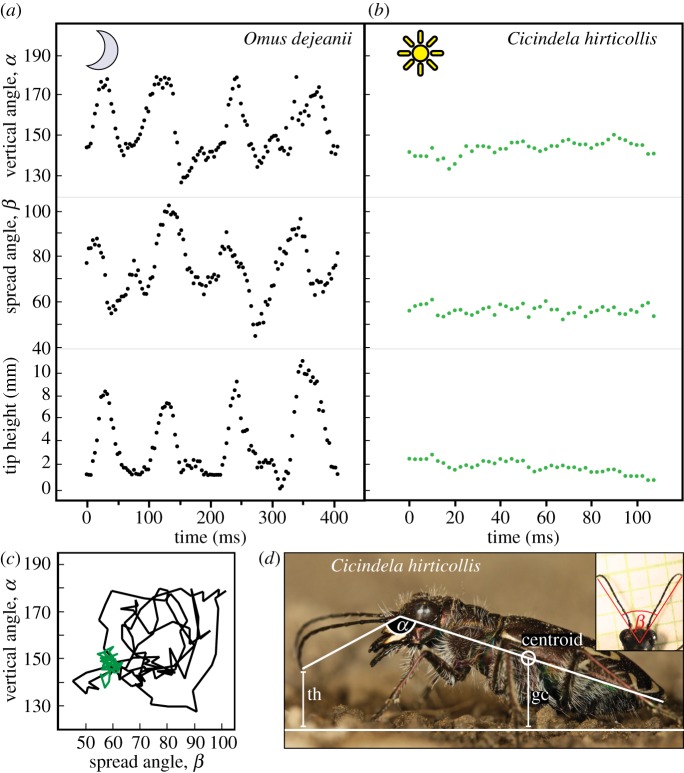
Additionally, lateral and dorsal views of straight runs by four nocturnally active tiger beetles, O. dejeanii, were filmed at 300 fps without placing obstacles in the arena. Only sprints perpendicular to the lateral camera angle were analysed. In this footage from four nocturnal beetles and in corresponding recordings of five diurnal C. hirticollis, we measured antenna positions during two step cycles using ImageJ v. 1.46r (Wayne Rasband, NIH, USA). Three measurements were taken from each frame (figure 1): the vertical angle (α) between longitudinal body axis and antenna; the horizontal angle (β) between the two antennae and the antennal tip height above the ground (th). Alpha and th were determined from the antenna in foreground of the digital image.
Figure 1.
Antennal positions during two-step cycles in running tiger beetles (note different timescales in (a) and (b)). Top row: vertical included angle α between the longitudinal body axis and antennal tip. Middle row: horizontal spread angle β between lines from antenna tip through the basal segment. Bottom row: height of the antenna tip above ground (th). (a) Nocturnal O. dejeanii move antennae up and down and from medial to lateral at twice the stepping frequency. (b) Diurnal C. hirticollis holds the antennae in a rigid, downward posture with the tips close to the ground. (c) Vertical antenna angle α of O. dejeanii and C. hirticollis plotted as a function of the horizontal spread angle β. The nocturnal beetles carry out wide elliptical movements, while the diurnal beetle holds its antennae in a steady position. (d) C. hirticollis, measured angles and tip height above ground overlaid. The centroid of the beetle's body image was determined automatically by image analysis (see Methods) and provided a measure of ground clearance of the body (gc). Inset shows antennal spread angle β. (Online version in colour.)
(d). Analyses
Video recordings were analysed both manually and via automated video motion tracking in a custom MATLAB program (see below). In all runs, we determined whether or not obstacles were successfully surmounted without ‘face planting’, i.e. did the beetle move smoothly over the obstacle rather than coming to an abrupt stop upon running headlong into the obstacle. For each set of 10 replicates, numbers of successes with antenna contact, successes without antenna contact, and failures were determined and averaged across the beetles in the group. Effects of individual, vision, antenna, obstacle contrast and obstacle height on number of ‘face plants’ were then tested with a linear-mixed model (standard least squares, REML fitting method) as implemented in JMP 10.0.2d1 (SAS Institute Inc., 2012). Individual was treated as a random effect, and antenna, vision, obstacle contrast and height as fixed nominal effects. First, all complete factor combinations were tested. Then, non-significant interactions (p > 0.05) were omitted and pooled into the error term in order to produce a more parsimonious model.
A customized MATLAB program (MATLAB R2012b, Computer Vision System Toolbox v. 5.1) developed in our laboratory, was used to analyse high-speed recordings frame-by-frame in each run. Using blob detection methods, the program determined the position and dimensions of the beetle in dorsal and lateral views, and calculated its x-, y- and z-coordinates in the arena, its velocity, the body pitch angle of the longitudinal axis against the ground and the distance from the beetle's centre of mass (centroid) to the ground (ground clearance, gc). We calculated means of velocity, pitch angle and ground clearance for controls, blinded and antennectomized beetles from runs without obstacles, in order to describe treatment effects on behaviour. Group means were compared using Kruskal–Wallis tests, and where significant these were followed by comparisons with the control using the Steel test. Potential differences in posture and velocity in response to obstacle height during obstacle approach, i.e. before contact, were tested using Kruskal–Wallis by obstacle height and treatment group.
We then investigated changes in these metrics within the space of one body length before an obstacle edge. Distance at which first contact of any body part with the obstacle occurred was determined manually, and means of antennectomized and blinded groups were compared with the control using Kruskal–Wallis followed by the Steel test. Then, video tracking data of each run within a treatment group were offset, so that traces started at the same y-level (to the average of the first 4 mm of all runs). Then, tracker measurements of distance to the obstacle were binned in 100 μm steps, and postural data for each treatment were averaged across runs.
3. Results
(a). Antennal posture in nocturnal and diurnal species
When running on a straight path, nocturnal O. dejeanii moves its antennae up and down and from medial to lateral at twice its stepping frequency (figure 1a). This results in approximately circular motion of the antennal tips, typical of other nocturnal or poorly sighted insects. Mean vertical angle α of the antenna is 149.1 ± 11.9°, resulting in tip height (th) of 4.6 ± 2.7 mm (N = 4 beetles, frame-by-frame measurements during two step cycles at 300 fps, means weighted by number of captured frames). Mean horizontal spread angle β of the antennae is 83.9 ± 10.4°.
During ranging or predatory runs in their habitat, or straight runs in a laboratory arena, members of the diurnal genus Cicindela hold their antennae in a distinctive, rigid posture: straightforward and angled slightly downward, with the distal tips always abruptly bent down almost touching the ground (figure 1b–d). In the field, there does not appear to be variation in relative running speeds: members of Cicindela either run at top speed or stand still (personal observation). The beetles are able to move their antennae into other postures, for instance when grooming them by pulling through the forelegs, or when the beetles are handled. During runs in the straight arena, the mean vertical angle α of the antennae is 139.9° ± 3.6°, resulting in antennal tip height (th) of 1.63 ± 0.63 mm (N = 5 beetles, frame-by-frame measurements during two step cycles each at 400 fps, means weighted by number of frames). The horizontal spread angle β of the antennae is 60.6° ± 2.5°. The shape and relatively rigid posture of the antennae of diurnal beetles relative to the more typical antennal behaviour of the nocturnal beetles led us to hypothesize that during running diurnal tiger beetles may use their antennae for a specialized mechanosensory purpose, namely the detection of obstacles.
(b). Obstacle negotiation
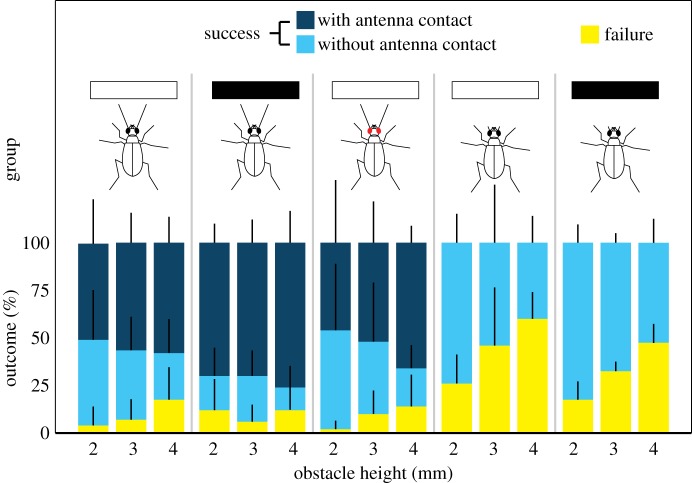
To investigate the roles of vision and antennal mechanosensation in obstacle negotiation by a diurnal tiger beetle, we filmed diurnal C. hirticollis sprinting over obstacles. We recorded whether obstacles were successfully surmounted and whether antennae made contact or not. Unimpaired beetles with low-contrast obstacles (2, 3 or 4 mm high) were used as baseline (20 beetles, 10 runs per obstacle height, 600 runs). Face plants, i.e. contact of the head with the side of the obstacle, were uncommon among unimpaired beetles (control), but became slightly more frequent with increasing obstacle height (figure 2). The same 20 beetles were then assigned to one of four treatment groups (i.e. five beetles per group): unimpaired with high-contrast obstacles (i), blinded (ii), antennectomized with low-contrast obstacles (iii) and antennectomized with high-contrast obstacles (iv). Linear-mixed-model analysis (R2 = 0.709 and the electronic supplementary material, table S1) revealed antennal presence (F = 83.91, d.f. = 1, p < 0.0001) and obstacle height (F = 22.33, d.f. = 2, p < 0.0001) as the most significant factors for successful obstacle negotiation, whereas changes to vision (F = 0.12, d.f. = 1, p > 0.7) and obstacle contrast (F = 0.21, d.f. = 1, p > 0.6) had no effect.
Figure 2.
Outcomes of obstacle runs (means ± s.d., Ncontrol = 20 beetles, other groups N = 5 beetles; 10 runs per obstacle height for each beetle). ‘Success’ means surmounting of the obstacle without any part of the head coming into contact. ‘Failure’ means that the head contacted the obstacle, stopping the beetle. White and black rectangles indicate low- and high-contrast treatments, respectively. Beetle icons represent unimpaired, blinded and antennectomized beetles. (Online version in colour.)
Low obstacles (2 mm) were often negotiated without antennal contact, but across all obstacles, the antennae were the most frequent first point of contact. Typically, the flexible curved ends of the antennae that contact the obstacle first are bent back and then slide over the edge (see the electronic supplementary material, movie S1). Unimpaired beetles faced with high-contrast obstacles showed the highest incidence of antennal contact (figure 2), and had a similar low incidence of face plants as the controls and the blind group. By contrast, after antennectomy, the incidence of face plants increased up to 60 ± 14.1%. Taken together, these results indicate that antennae are both necessary and sufficient to provide the running beetle with spatial information about its environment and that vision is neither sufficient nor necessary.
(c). Postural changes during obstacle approach
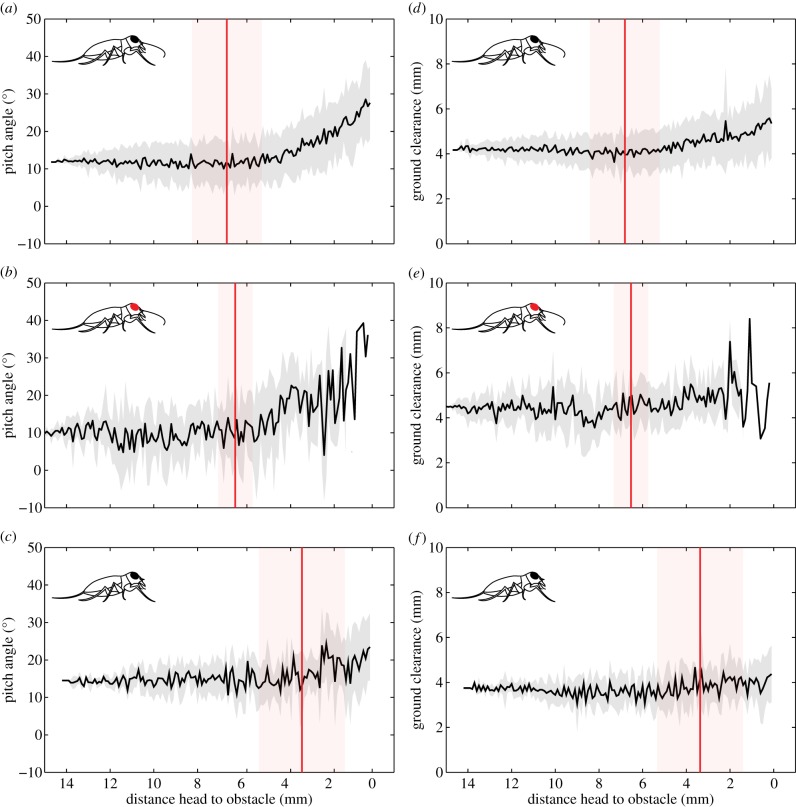
In unobstructed runs, antennectomized beetles showed a small decrease in average ground clearance compared with controls (figure 3). During obstacle approach (i.e. before contact), differences in posture or velocity in response to obstacle height were not observed in any group (Kruskal–Wallis tests by obstacle height and treatment, all p-values > 0.075). The beetle-to-obstacle distance at which the first contact with any body part occurred was 6.83 ± 1.60 mm in controls, 6.54 ± 0.79 mm in blind and was 3.48 ± 1.97 mm in antennectomized beetles (control significantly different from antennectomized but not from blind, Kruskal–Wallis H = 12.49, d.f. = 2, p = 0.002, Steel, p = 0.0015). Body pitch angle increased after obstacle contact in all groups (figure 4a–c). Body ground clearance increased as well, but to a lesser extent (figure 4d–f).
Figure 3.
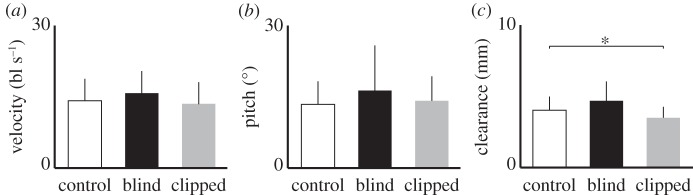
Velocity, pitch angle and ground clearance of beetles during runs on flat ground, before and after covering eyes or clipping antennae (mean ± s.d. control: N = 20 beetles, n = 225 runs; blind: N = 5, n = 46; clipped: N = 10, n = 75); (a,b) treatments had no effect on velocity and body pitch angle (c) antennectomized beetles slightly decreased ground clearance (Kruskal–Wallis test (H = 8.43, d.f. = 2, p = 0.015) followed by comparison with control using Steel method (p = 0.023)).
Figure 4.
(a–c) Pitch angle and (d–f) ground clearance as function of distance to the ‘high’ obstacle (4 mm). Black line represents mean of all runs in a treatment group (±s.d., grey overlay). Individual run data were offset so that traces started at the same y-value (offset to the mean of the first 4 mm of all runs in the group). Vertical line is the mean of the distance at first contact with the obstacle (±s.d., shaded overlay). Top panels: control group (N = 20 beetles, n = 172 runs), middle panels: blind group (N = 5 beetles, n = 34 runs), bottom panels: antennectomized group (N = 5 beetles, n = 33 runs). (Online version in colour.)
4. Discussion
We found that during locomotion, antennae of nocturnal tiger beetles perform roughly circular motions that are typical of active mechanosensory searching by other nocturnal or poorly sighted insects, such as cockroaches [13,22] and walking sticks [23]. While the range of their sensory volume is limited to the length of the antennae and the mechanosensory spatial information is processed serially [23], this is the best they can achieve with sparse photic information. On the other hand, diurnal tiger beetles are active in intensely bright environments and have excellent vision, but we show here that they, nevertheless, rely on mechanosensation and not on vision to negotiate obstacles. In that respect, the beetles behave more like animals that are active in low-light regimes. A major difference, however, is that animals well adapted to low-light regimes actively move their mechanosensors to acquire spatial information about their environment. Diurnal tiger beetles, however, hold their antennae rigidly forward and close to the ground while running. We show here that antennae alone are necessary and sufficient for reliable obstacle negotiation. Antennectomized beetles collided with up to 60% of obstacles, whereas loss of vision did not result in more frequent collisions compared with intact control beetles. In runs without obstacles, antennectomized beetles slightly decreased their ground clearance, which likely represents an attempt to bring the now missing antennae in contact with the ground, equivalent to the behaviour of antennectomized cockroaches when wall-following [24]. Such postural compensation for experimental sensory deficit is also seen in other sensory modalities (vision: [25] and proprioception: [26]).
(a). Utilization of visual and mechanosensory spatial information for obstacle negotiation
With interommatidial angles of about 1° in the horizontal acute streak [18], members of the genus Cicindela may have sufficient spatial resolution to identify obstacles straight ahead in the optic flow field generated by running. In principle, the pole of the flow field during straightforward motion has zero image velocity [27]. However, tiger beetles run with an alternating tripod gait that generates about ± 5° sinusoidal wobble in the yaw plane, resulting in image velocities that can reach 500 deg s−1 [28]. The critical velocity of C. hirticollis photoreceptors is about 75 deg s−1 [29], after which image contrast is decreased and higher spatial frequencies, for example, small details such as chased prey, can no longer be resolved. The diurnal tiger beetles literally operate in the ‘collision mode’ (sensu [30]), in which the sensory volume is much smaller than their locomotor control volume. Eyes are energetically costly to maintain owing to the large number of photoreceptors that consume large amounts of ATP [31]. This cost is exacerbated in eyes with high temporal resolution, and therefore high excitation frequencies. Tiger beetle photoreceptors are large and have fast peak times, indicating high temporal resolution [18]; thus, using a simple, passive mechanosensory alternative to constant real-time analysis of fast optic flow while running may provide an advantage in terms of energy consumption.
Diurnal tiger beetles of the genus Cicindela are a phylogenetically diverse group [32], yet they share similar lifestyle, posture and antennal morphology, with the antennae held rigidly forward in a static ‘V’ shape and downwards, with the terminal segments of the flagellum bent towards the ground. While the beetle runs, they are held at an almost constant height above ground, which enables the antennal tips to brush over elevations in the surface topography. The shape and flexibility of the tips allow them to make contact with such hurdles, briefly snag on them, and slide spring-like over the obstacle edge, which evidently provides sufficient information about the obstacle's height and shape, sufficiently early, to initiate postural changes of pitching up and increasing ground clearance that allow for uninterrupted negotiation of the obstacle. Cockroaches engaged in fast running along a wall also hold the antennae in a relatively fixed horizontal separation of about 60° with the ipsilateral antenna in contact with the wall about 75% of the time [24,33]. Thus, antennal movements in the cockroach are somewhat context-dependent: during open field exploration, they are actively moved to acquire wide-field spatial information with low spatio-temporal resolution [13], but during thigmotactic escape, they are held rigidly.
By contrast, diurnal tiger beetles do not appear to show context-dependent movement of the antennae for acquisition of spatial information. Regardless of whether the beetle is stationary, walking slowly over uneven terrain, such as erosional crevasses, ranging through its habitat or chasing prey, the antennae are rigidly held forward with the tips close to, but not in contact with, the substrate. Thus, in contrast to actively moved ‘feelers’ that may spend a large amount of time away from the substrate, the detection rate for objects in front of the beetle is maximized using static antennae. The biomechanical properties of tiger beetle antennae remain to be investigated, but their distal-curved shape might be particularly suited to the task of detecting low obstacles during running—in a study on alternative shapes of walking canes for blind people, canes with tips curved downwards were gauged by participants to provide improved sensory input with respect to obstacle detection [34]. Slower, herbivorous beetles also depend on antennae, rather than on vision, during locomotion. Leaf beetles (Chrysomelidae) angle their body upwards when antennae encounter an obstacle [35]. The visual systems of these beetles, however, do not provide enough resolution to resolve obstacle details. The authors predicted that the importance of the antennae for terrestrial locomotion would decrease with increased visual acuity. Diurnal cicindelids present an interesting departure from this hypothesis.
Spatial information for obstacle negotiation by some insects is acquired by both vision and mechanoreception [36]. In some cases, the multimodal information seems to be redundant. Locusts walking across a horizontal ladder [37] can avoid gaps and place their feet appropriately on rungs using spatial information from antennae or the ipsilateral eye. The error rate increases only when both modalities are impaired. Other insects, however, use multimodal sensory information to bias obstacle negotiating strategies. Cockroaches use visual information from ocelli [11] or compound eyes [36] to modulate the direction of obstacle avoidance after the antennae have provided mechanosensory information about the obstacle's position and size. This is not the case with diurnal tiger beetles. Even with the tallest obstacles of high visual contrast, the proportion of individuals face planting on the edge of the obstacle increased fourfold when the antennae were removed.
(b). Implications for other visual systems operating in fast optic flow
Many other animals move at high linear and angular velocities that may induce significant visual motion blur throughout much of their visual field. It is likely more pronounced in animals with acute vision, i.e. those with denser photoreceptor mosaics, because images move faster across the narrower visual fields of individual photoreceptors [3]. Thus, paradoxically, their good vision may not be very useful during such pursuit except to keep the image of the prey in the pole of the optic flow field. During the pursuit, spatial information about their surroundings may be supplied by another sensory modality, such as mechanosensation, that is not so affected by high velocities. This begs the question of how common multimodal input is during pursuits by other fast-moving animals. For instance, predatory blind fish living in caves rely on lateral line information to map space and avoid obstacles, but sighted conspecifics living in surface waters use vision during slow exploration of obstacles in their environments [38]. It is an open question whether these and other fast-moving animals are able to rely only on vision during fast pursuit or whether they also compensate with a short-range sensory modality to avoid obstacles.
The solution that diurnal tiger beetles have evolved to deal with obstacle detection in the face of overwhelming visual motion blur could prove highly relevant for application in autonomous vehicles. One of the earliest autonomous robots, Shakey [39], used passive antennae, so-called bump detectors, that were not unlike the tiger beetle's rigidly held antennae. When they made contact, they deformed and signalled Shakey to change its behaviour. It simply stopped, then reversed direction. More modern robots that use bioinspired antennae to acquire spatial information about the environment use them in passive [40] or active [41] modes, and the behavioural output is more flexible. They allow the robot to avoid collision and negotiate the obstacle while continuing forward movement.
Analysis of surface topography is a basic and universal challenge for all autonomous vehicles. Compared with mechanosensory acquisition, energy-based systems using sonar or visual sensors require significant computational resources for real-time analysis [42]. This not only ties up valuable CPU cycles, but also by extension leads to higher power consumption. This requires bigger batteries, reduces payload and limits miniaturization. For example, NASA's Curiosity rover performs obstacle detection with a dedicated computer controlling eight hazard avoidance cameras (‘Hazcams’) that produce a stereoscopic three-dimensional surface map of the immediate surroundings, but the rover only achieves a top speed of 3.8 cm s−1 [43]. While safety is a higher priority than speed in this case, comparatively simple beetle-inspired fixed angle-curved antennae could provide a low-cost solution to collision avoidance while enabling higher speeds in autonomous vehicles.
Acknowledgements
We thank Jane Wang for use of the Phantom camera, Françoise Vermeylen for statistical consultation, David Duneau for the image of C. hirticollis in figure 1d, and an anonymous reviewer for cogent remarks about a previous draft of the manuscript.
Data accessibility
Data are available at www.datadryad.org (doi:10.5061/dryad.d9b2t).
Funding statement
This work was supported by NSF award IOS 0950688 to C.G.
References
- 1.Jander R. 1975. Ecological aspects of spatial orientation. Annu. Rev. Ecol. Syst. 6, 171–188 (doi:10.1146/annurev.es.06.110175.001131) [Google Scholar]
- 2.Dusenbery DB. 1992. Sensory ecology: how organisms acquire and respond to information. New York, NY: WH Freeman [Google Scholar]
- 3.Land MF, Nilsson DE. 2012. Animal eyes, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- 4.Warrant EJ, Kelber A, Gislén A, Greiner B, Ribi W, Wcislo WT. 2004. Nocturnal vision and landmark orientation in a tropical halictid bee. Curr. Biol. 14, 1309–1318 (doi:10.1016/j.cub.2004.07.057) [DOI] [PubMed] [Google Scholar]
- 5.Klump GM. 2000. Sound localization in birds. In Springer handbook of auditory research (eds Dooling RJ, Fay RR, Popper AN.), pp. 249–307 New York, NY: Springer [Google Scholar]
- 6.Nelson ME, MacIver MA. 2006. Sensory acquisition in active sensing systems. J. Comp. Physiol. A 192, 573–586 (doi:10.1007/s00359-006-0099-4) [DOI] [PubMed] [Google Scholar]
- 7.Culver DC, Kane TC, Fong DW. 1995. The evolution of Gammarus minus. In Adaptation and natural selection in caves, pp. 1–235 New York, NY: Harvard University Press [Google Scholar]
- 8.Bauer T, Kredler M. 1993. Morphology of the compound eyes as an indicator of life-style in carabid beetles. Can. J. Zool. 71, 799–810 (doi:10.1139/z93-105) [Google Scholar]
- 9.Hartmann MJ. 2001. Active sensing capabilities of the rat whisker system. Auton. Robots 11, 249–254 (doi:10.1023/A:1012439023425) [Google Scholar]
- 10.Staudacher EM, Gebhardt M, Dürr V. 2005. Antennal movements and mechanoreception: neurobiology of active tactile sensors. In Advances in insect physiology (ed. SJ Simpson), vol. 32, pp. 49–205 Oxford UK: Academic Press. [Google Scholar]
- 11.Harley CM, English BA, Ritzmann RE. 2009. Characterization of obstacle negotiation behaviors in the cockroach, Blaberus discoidalis. J. Exp. Biol. 212, 1463–1476 (doi:10.1242/jeb.028381) [DOI] [PubMed] [Google Scholar]
- 12.Baba Y, Tsukada A, Comer CM. 2010. Collision avoidance by running insects: antennal guidance in cockroaches. J. Exp. Biol. 213, 2294–2302 (doi:10.1242/jeb.036996) [DOI] [PubMed] [Google Scholar]
- 13.Okada J, Toh Y. 2004. Spatio-temporal patterns of antennal movements in the searching cockroach. J. Exp. Biol. 207, 3693–3706 (doi:10.1242/jeb.01201) [DOI] [PubMed] [Google Scholar]
- 14.Dürr V, König Y, Kittmann R. 2001. The antennal motor system of the stick insect Carausius morosus: anatomy and antennal movement pattern during walking. J. Comp. Physiol. A 187, 131–144 (doi:10.1007/s003590100183) [DOI] [PubMed] [Google Scholar]
- 15.Hebets EA. 2002. Relating the unique sensory system of amblypygids to the ecology and behavior of Phrynus parvulus from Costa Rica (Arachnida, Amblypygi). Can. J. Zool. 80, 286–295 (doi:10.1139/z02-006) [Google Scholar]
- 16.Kuster JE. 1979. Comparative structure of compound eyes of Cicindelidae and Carabidae (Coleoptera): evolution of scotopy and photopy. Quaest. Entomol. 15, 297–333 [Google Scholar]
- 17.Friederichs HF. 1931. Beiträge zur Morphologie und Physiologie der Sehorgane der Cicindelinen (Col.). Z. Morph. u. Okol. Tiere 21, 1–172 (doi:10.1007/BF00406496) [Google Scholar]
- 18.Layne JE, Gilbert C. 2006. Vision on the run: suboptimal spatio-temporal resolution of natural images by tiger beetles. In Neuroscience 2006: 36th Ann. Meeting of Society for Neuroscience, 14–18 October, Atlanta, GA, abstract 351.10/X5. [Google Scholar]
- 19.Gilbert C. 1997. Visual control of cursorial prey pursuit by tiger beetles (Cicindelidae). J. Comp. Physiol. A 181, 217–230 (doi:10.1007/s003590050108) [Google Scholar]
- 20.Pearson DL, Vogler AP. 2001. Tiger beetles: the evolution, ecology, and diversity of the Cicindelids. Ithaca, NY: Cornell University Press [Google Scholar]
- 21.Kamoun S, Hogenhout SA. 1996. Flightlessness and rapid terrestrial locomotion in tiger beetles of the Cicindela L. subgenus Rivacindela van Nidek from saline habitats of Australia (Coleoptera: Cicindelidae). Coleopts. Bull. 50, 221–230 [Google Scholar]
- 22.Bell WJ. 1991. Searching behaviour. The ecology of finding resources. London, UK: Chapman, Hall [Google Scholar]
- 23.Krause AF, Winkler A, Dürr V. 2012. Central drive and proprioceptive control of antennal movements in the walking stick insect. J. Physiol. (Paris) 107, 116–129 (doi:10.1016/j.jphysparis.2012.06.001) [DOI] [PubMed] [Google Scholar]
- 24.Camhi JM, Johnson EN. 1999. High-frequency steering maneuvers mediated by tactile cues: antennal wall-following in the cockroach. J. Exp. Biol. 202, 631–643 [DOI] [PubMed] [Google Scholar]
- 25.Mast SO. 1924. The process of photic orientation in the robber-fly, Proctacanthus philadelphicus. Am. J. Physiol. 68, 262–279 [Google Scholar]
- 26.Gilbert C, Kim MP. 2007. Effects of male age and cervical proprioceptors on sexual aerial pursuit by male flesh flies, Neobellieria bullata (Diptera: Sarcophagidae). J. Insect Behav. 20, 427–435 (doi:10.1007/s10905-007-9088-x) [Google Scholar]
- 27.Koenderink JJ. 1986. Optic flow. Vision Res. 26, 161–179 (doi:10.1016/0042-6989(86)90078-7) [DOI] [PubMed] [Google Scholar]
- 28.Haselsteiner A, Gilbert C, Wang ZJ. Submitted. Closed loop visual control of pursuit by the tiger beetle, Cicindela hirticollis Say (Carabidae: Cicindelinae). J. R. Soc. Interface.
- 29.Layne JE, Chen PW, Gilbert C. 2006. The role of target elevation in prey selection by tiger beetles (Carabidae: Cicindela spp.). J. Exp. Biol. 209, 4295–4303 (doi:10.1242/jeb.02529) [DOI] [PubMed] [Google Scholar]
- 30.Snyder JB, Nelson ME, Burdick JW, MacIver MA. 2007. Omnidirectional sensory and motor volumes in electric fish. PLoS Biol. 5, e301 (doi:10.1371/journal.pbio.0050301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 (doi:10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 32.Vogler A. 1996. A molecular phylogeny of the tiger beetles (Cicindelidae): congruence of mitochondrial and nuclear rDNA data sets. Mol. Phylogenet. Evol. 6, 321–338 (doi:10.1006/mpev.1996.0083) [DOI] [PubMed] [Google Scholar]
- 33.Cowan NJ, Lee J, Full RJ. 2006. Task-level control of rapid wall following in the American cockroach. J. Exp. Biol. 209, 1617–1629 (doi:10.1242/jeb.02166) [DOI] [PubMed] [Google Scholar]
- 34.Schellingerhout R, Bongers RM, Van Grinsven R, Smitsman AW, Van Galen GP. 2001. Improving obstacle detection by redesign of walking canes for blind persons. Ergonomics 44, 513–526 (doi:10.1080/00140130120830) [DOI] [PubMed] [Google Scholar]
- 35.Pelletier Y, McLeod CD. 1994. Obstacle perception by insect antennae during terrestrial locomotion. Physiol. Entomol. 19, 360–362 (doi:10.1111/j.1365-3032.1994.tb01063.x) [Google Scholar]
- 36.Ritzmann RE, et al. 2012. Deciding which way to go: how do insects alter movements to negotiate barriers? Front. Neurosci. 6, 97 (doi:10.3389/fnins.2012.00097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niven JE, Buckingham CJ, Lumley S, Cuttle MF, Laughlin SB. 2010. Visual targeting of forelimbs in ladder-walking locusts. Curr. Biol. 20, 86–91 (doi:10.1016/j.cub.2009.10.079) [DOI] [PubMed] [Google Scholar]
- 38.Sutherland L, Holbrook RI, Burt De Perera T. 2009. Sensory system affects orientational strategy in a short-range spatial task in blind and eyed morphs of the fish, Astyanax fasciatus. Ethology 115, 504–510 (doi:10.1111/j.1439-0310.2009.01630.x) [Google Scholar]
- 39.Nilsson NJ. 1984. Shakey the robot. Tech note 323. Menlo Park, CA: SRI International [Google Scholar]
- 40.Cowan NJ, Ma EJ, Cutkosky M, Full RJ. 2005. A biologically inspired passive antenna for steering control of a running robot. In Robotics research (eds Dario P, Chatila R.), pp. 541–550 Berlin, Heidelberg: Springer [Google Scholar]
- 41.Lewinger WA, Harley CM, Ritzmann RE, Branicky MS, Quinn RD. 2005. Insect-like antennal sensing for climbing and tunneling behavior in a biologically-inspired mobile robot. Proc. IEEE Int. Conf. Robot. Autom. 4176–4181 (doi:10.1109/ROBOT.2005.1570761) [Google Scholar]
- 42.Manduchi R, Castano A, Talukder A, Matthies L. 2005. Obstacle detection and terrain classification for autonomous off-road navigation. Auton. Robots 18, 81–102 (doi:10.1023/B:AURO.0000047286.62481.1d) [Google Scholar]
- 43.Christensen P.2012. Mars science laboratory: eyes and other senses. See http://mars.jpl.nasa.gov/msl/mission/rover/eyesandother/ .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at www.datadryad.org (doi:10.5061/dryad.d9b2t).