Abstract
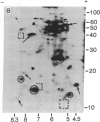
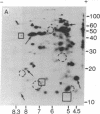
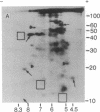
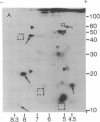
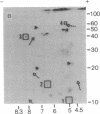
Total polysomal poly(A)+ RNA of Novikoff hepatoma and of 18-hr regenerating rat liver were compared by analysis of their in vitro translational products on two-dimensional isofocusing/sodium dodecyl sulfate gels. This technique resolved the translated proteins sufficiently to permit detection of quantitative and some qualitative differences between the two mRNA populations. Excess cDNA from regenerating liver or Novikoff hepatoma, covalently linked to cellulose, was used to adsorb the complementary mRNA sequences from Novikoff hepatoma or regenerating liver. As shown by two-dimensional gel electrophoresis, the translated products of the bound mRNA fractions contained proteins common to both tissues. Novikoff hepatoma mRNA which did not bind to regenerating liver cDNA was enriched in sequences encoding for proteins 11/5.1, 15/6.8, 40/8.2, and 65/5.1 (shown as molecular weight/pI). These polypeptides were not detectable in the translational products of regenerating liver mRNA. Regenerating liver mRNA that was not bound to Novikoff hepatoma cDNA was enriched in sequences coding for proteins 12.5/4.9, 13.5/7.4, 17/8.2, 24/5.5, and 46/6.4; these proteins were not found in the translational pattern from Novikoff hepatoma. These results show that adsorption of mRNA to solid-phase cDNA provides a valuable technique for differentiating mRNA species in related tissues and for corresponding enrichment of these specific mRNAs.
Keywords: cancer genes, gene control mechanisms, tissue phenotypes
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Schimke R. T. Partial purification of the ovalbumin gene. Cell. 1976 Mar;7(3):331–338. doi: 10.1016/0092-8674(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Kabat D. Potentiation of hemoglobin messenger ribonucleic acid. A step in protein synthesis initiation involving interaction of messenger with 18 S ribosomal ribonucleic acid. J Biol Chem. 1975 Aug 10;250(15):6085–6092. [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Leder P., Ross J., Gielen J., Packman S., Ikawa Y., Aviv H., Swan D. Regulated expression of mammalian genes: globin and immunoglobulin as model systems. Cold Spring Harb Symp Quant Biol. 1974;38:753–761. doi: 10.1101/sqb.1974.038.01.080. [DOI] [PubMed] [Google Scholar]
- Levy S., Aviv H. Quantitation of labeled globin messenger RNA by hybridization with excess complementary DNA covalently bound to cellulose. Biochemistry. 1976 May 4;15(9):1844–1847. doi: 10.1021/bi00654a009. [DOI] [PubMed] [Google Scholar]
- Lin A., Collatz E., Wool I. G. Micro-scale two-dimensional polyacrylamide gell electrophoresis of ribosomal proteins. Mol Gen Genet. 1976 Feb 27;144(1):1–9. doi: 10.1007/BF00277296. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Wahli W., Weber R. Quantitation of vitellogenin messenger RNA in the liver of male Xenopus toads during primary and secondary stimulation by estrogen. Cell. 1977 May;11(1):213–221. doi: 10.1016/0092-8674(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Venetianer P., Leder P. Enzymatic synthesis of solid phase-bound DNA sequences corresponding to specific mammalian genes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3892–3895. doi: 10.1073/pnas.71.10.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., Lingrel J. B. Purification of biologically active globin mRNA using cDNA-cellulose affinity chromatography. J Biol Chem. 1977 Jan 25;252(2):457–463. [PubMed] [Google Scholar]
- Wu B. C., Rao M. S., Gupta K. K., Rothblum L. I., Mamrack P. C., Busch H. Evidence for coupled synthesis of mRNA for ribosomal proteins and rRNA. Cell Biol Int Rep. 1977 Jan;1(1):31–44. doi: 10.1016/0309-1651(77)90007-8. [DOI] [PubMed] [Google Scholar]
- Young B. D., Birnie G. D. Complexity and specificity of polysomal poly(A+) RNA in mouse tissues. Biochemistry. 1976 Jun 29;15(13):2823–2829. doi: 10.1021/bi00658a019. [DOI] [PubMed] [Google Scholar]