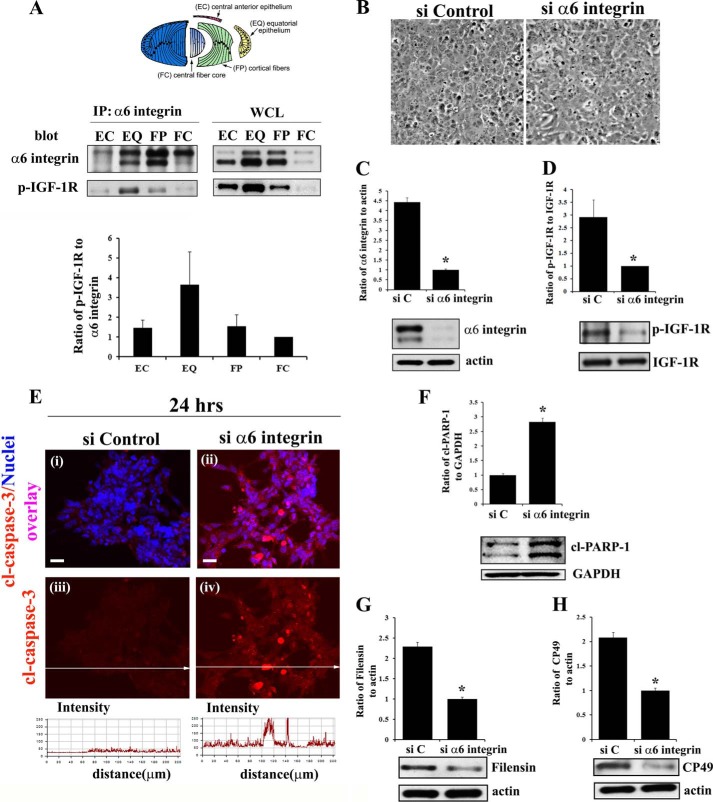
FIGURE 4.
α6 integrin transactivation of IGF-1R maintains low level caspase-3 activity for its role in signaling differentiation initiation of lens epithelial cells. A, diagram of the microdissected lens areas (top panel); co-immunoprecipitation (IP, α6 integrin; blot, α6 integrin and activated (phosphorylated) IGF-1R (p-IGF-1R)) analysis in differentiation specific zones of E10 chick embryo lenses, obtained by microdissection. α6 integrin was immunoprecipitated with an α6A integrin-specific polyclonal antibody because this is the predominant isoform associated with lens differentiation (47). Immunoprecipitates and whole cell lysates (WCL) were immunoblotted with a monoclonal antibody to the α6 integrin extracellular domain that recognizes two bands, the cleaved (lower band) and uncleaved (upper band) form of α6 integrin as described previously (47, 62). Results of three independent experiments were quantified and represented graphically as a ratio of p-IGF1R/α6 integrin (both cleaved and uncleaved bands), showing that IGF-1R is activated specifically in the α6 integrin signaling complexes in the EQ zone. B–H, α6 integrin was knocked down using a siRNA approach (si α6 integrin) in primary lens cells in culture. Control cultures were treated with non-targeting siRNA (siControl/siC). B, phase images of lens epithelial cells following siRNA knockdown of α6 integrin compared with siControl. C, immunoblot for α6 integrin showing siα6 integrin effectively knocked down α6 integrin expression, and D, activation of IGF-1R (p-IGF-1R), without affecting expression of total IGF-1R. E, immunostaining for cleaved (activated) caspase-3 (red, i–iv), co-stained with TO-PRO-3 to detect nuclei (blue, i–ii), showed increased caspase-3 activation following α6 integrin knockdown. Fluorescence intensity of cl-caspase-3 was quantified by creating line scans (panels iii and iv, quantified below) across the field of cells using LSM Image Examiner, confirming increased caspase-3 activation in response to α6 integrin knockdown. F, immunoblot for the caspase-3 target cl-PARP-1. Immunoblot analysis was also performed for the lens differentiation specific proteins filensin (G) and CP49 (H). The results show that α6 integrin is necessary to maintain low levels of caspase-3 activity and inducing lens epithelial cell differentiation initiation. Densitometric analyses of immunoblots for α6 integrin, filensin, and CP49 were plotted as a ratio to actin; cl-PARP-1 as a ratio to GAPDH; and p-IGF-1R against total IGF-1R. For confocal imaging, z-stacks were collected and analyzed, and the data are presented as a single optical plane of 0.5 μm. Results are representative of at least three or more independent studies. Error bars represent S.E. *, p < 0.05, t test. Scale bar, 20 μm.