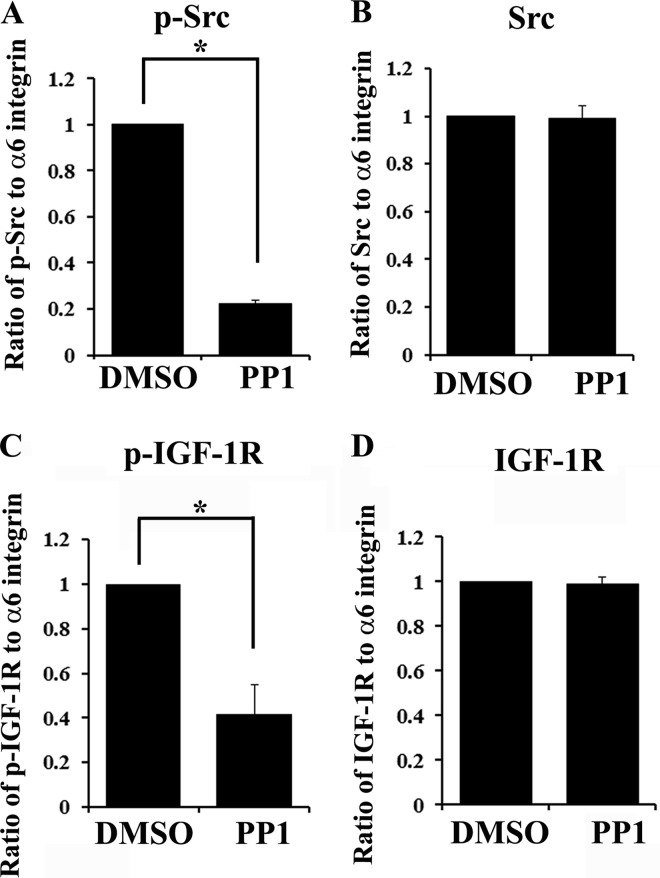
FIGURE 6.
Activation of IGF-1R in α6 integrin signaling complexes is dependent on the kinase activity of Src family kinases. Primary lens cells in culture were exposed to the highly specific Src family kinase inhibitor, PP1, or to the vehicle dimethyl sulfoxide (DMSO) for 4 h. Following PP1 treatment, cells were immunoprecipitated for α6 integrin and the immunoprecipitates were immunoblotted for α6 integrin and phospho-Src (p-Src) (A), Src (B), phospho-IGF-1R (p-IGF-1R) (C), and IGF-1R (D). A–D, graphical representation of densitometric analysis of co-immunoprecipitation studies showing the ratio of activated, p-Src/α6 integrin (A), total Src/α6 integrin (B), activated p-IGF-1R/α6 integrin (C), and total IGF-1R/α6 integrin (D). Data shows that blocking activation of Src kinases prevented α6 integrin transactivation of IGF-1R. Results shown are quantified from more than three independent studies. Error bars represent S.E. *, p < 0.05, t test.