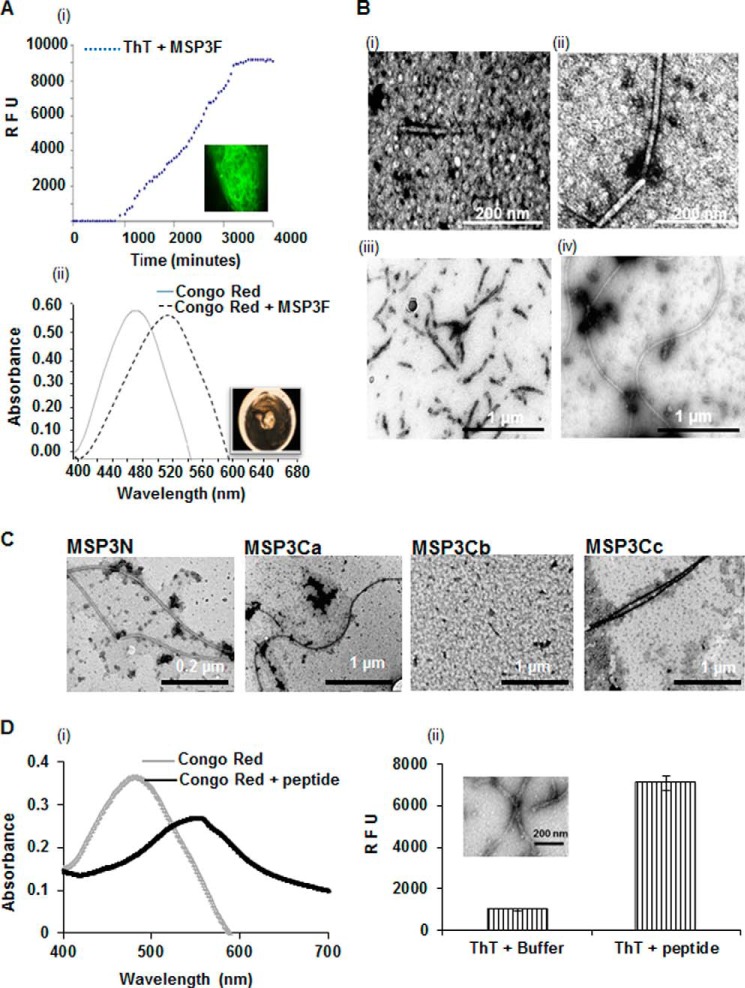
FIGURE 2.
Morphological characterization of MSP3 aggregates after different time points as determined by electron micrographs. A, kinetics of MSP3F fibril formation assessed by ThT fluorescence and Congo Red assay. ThT fluorescence for 0.5 mg/ml solution of MSP3F incubated at 25 °C shows an increase in fluorescence at lag phase followed by the exponential phase. Fluorescence images after completion of ThT kinetics of MSP3F showed fibrillar assemblies. Polymeric MSP3-bound Congo Red demonstrated maximum absorbance red-shifting from 488 to 540 nm and showing birefringence under polarized light. RFU, relative fluorescence unit. B, dependence on time of TEM images of 0.5 mg/ml solution of MSP3F, which was incubated at 25 °C in phosphate-buffered saline, pH 7.4. and sampled at t = 1 h (panel i), 30 h (panel ii), 55 h (panel iii), and 72 h (panel iv). Spherical intermediates observed initially transformed to truncated fibrils and finally long elongated unbranched fibrils. C, electron micrographs show fibril formation by MSP3N, MSP3Ca, and MSP3Cc under similar conditions. MSP3Cb showed no visible ordered morphology. D, amyloid characterization of synthetic peptide YILGW corresponding to MSP3(192–196) predicted as an aggregation-prone motif in MSP3 sequence. Peptide was positive in ThT binding assay and when incubated with Congo Red showed a characteristic red shift for Congo Red (panels i and ii). TEM micrographs (inset) depict the fibril-like morphology (panel ii).