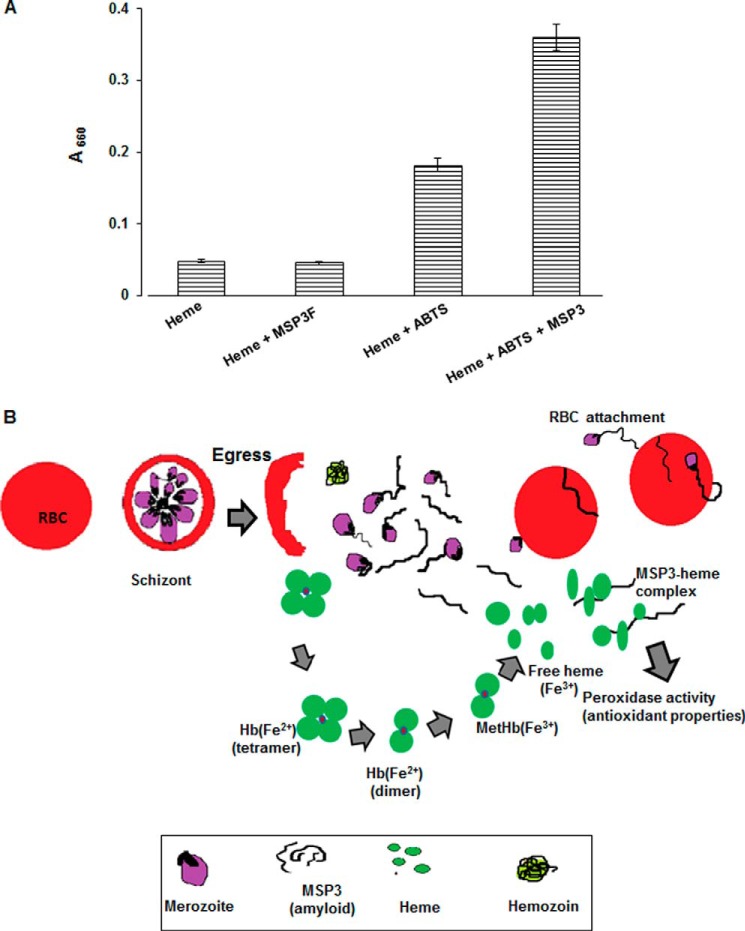
FIGURE 6.
MSP3-heme complex possesses peroxidase activity. A, ABTS assay detected 2-fold increase in absorption at 600 nm for MSP3-heme complex as compared with heme. No significant increase at 600 nm was observed for heme or MSP3F alone suggesting MSP3-heme to be an active species. B, model proposing MSP3 to act as a possible heme scavenger in its amyloid morphology. After completion of erythrocytic asexual life cycle by P. falciparum, unprocessed hemoglobin (Hb) along with merozoites are released in blood plasma. Released merozoites with attached MSP3 fibrillar structures reinvade red blood cells by associating RBC membrane with these structures. Heme iron in ferrous state (Fe2+) has high affinity for oxygen molecule that leads to formation of oxyhemoglobin. Autoxidation of oxyhemoglobin leads to production of ferric (Fe3+) hemoglobin (methemoglobin), ferryl hemoglobin, free heme, and several reactive oxygen species. They possess a potential threat as a possible cause of tissue damage and cell destruction. Haptoglobulin, a high affinity Hb binder along with α1-microglobulins, transferrin, albumins, hemopexins, and antioxidants such as vitamin E and ascorbic acid, cooperatively entrap free heme during hemolysis. However, in pathological conditions of malarial infection, these systems are not sufficient to combat oxidative stress to both host and parasite. Parasite expresses merozoite surface protein3 (MSP3) in abundance along with other unknown proteins that form amyloid fibril-like structures. These nascent heme molecules are entrapped by soluble MSP3 fibrils that modulate its peroxidase activity that might have antioxidant properties of some biological relevance.