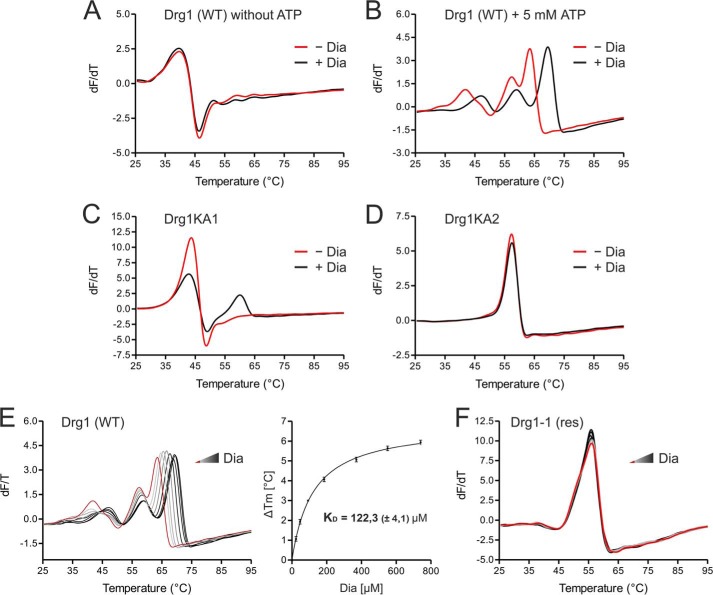
FIGURE 3.
Binding of diazaborine requires ATP loading into the D2 domain of Drg1. Binding of diazaborine (Dia) to Drg1 was investigated by DSF. 1.8 μm Drg1 (WT) or mutant variants thereof were incubated with different diazaborine concentrations in the absence or presence of ATP, and melting curves were recorded in the presence of SYPRO® Orange in a Rotor-Gene 6000. A and B, binding of diazaborine to Drg1 requires the presence of ATP. The purified Drg1 protein was incubated without (red curve) or with (black curve) 740 μm diazaborine in the absence (A) or presence (B) of 5 mm ATP. Addition of diazaborine resulted in a shift of the melting curve to higher temperatures in the presence of ATP, demonstrating binding of the inhibitor to the protein. C and D, diazaborine binding to Drg1 requires ATP loading into D2. Melting curves of Drg1 variants unable to bind ATP into the first (Drg1KA1 (C)) or the second (Drg1KA2 (D)) AAA domain were investigated in the presence of 5 mm ATP without (red curve) or with 740 μm diazaborine (black curve). Diazaborine treatment resulted in a change of the melting curve of Drg1KA1 (C) but not Drg1KA2 (D), showing that loading of ATP into D2 is necessary for diazaborine binding. E, diazaborine binding to Drg1 (WT) in the presence of 5 mm ATP was assessed quantitatively. Melting curves were recorded without diazaborine (red line) or in the presence of increasing diazaborine concentrations (0–740 μm; light gray to black gradient, left panel). Right panel, plotting of the increase in melting temperature (in relation to the untreated sample) over the drug concentration allows the estimation of a drug binding curve. The calculated KD (± S.D.) of three independent measurements is shown. F, the melting curve of the diazaborine-resistant protein Drg1-1 (res) shows no alterations even with 740 μm diazaborine, suggesting that the inhibitor does not bind to this protein.