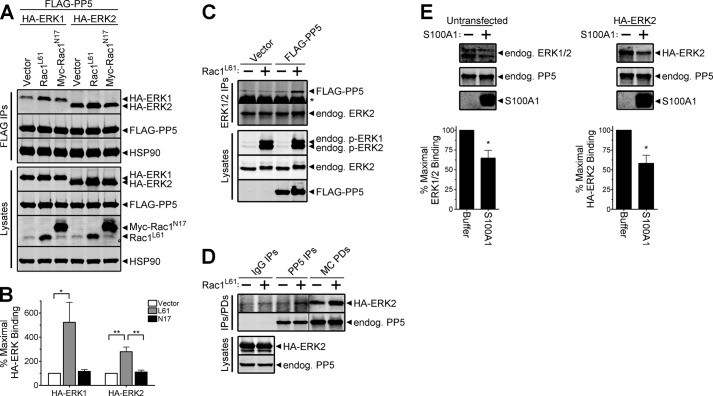
FIGURE 3.
Active Rac1 promotes assembly of PP5·ERK1 and PP5·ERK2 complexes. A, HEK-293FT cells were co-transfected with FLAG-PP5 and either HA-ERK1 or HA-ERK2 together with pcDNA3 (Vector), constitutively active Rac1 (Rac1L61), or dominant negative Rac1 (Myc-Rac1N17), as indicated. Western analysis of FLAG immune complexes (FLAG IPs) and cell lysates were performed using the HA, FLAG, HSP90, and Rac1 antibodies. B, quantification of the percentage of maximal HA-ERK binding normalized to the FLAG-PP5 signal in FLAG IPs, with binding in the vector samples set to 100. One-way analysis of variance identified a significant increase in PP5-ERK1 (F(2,6) = 6.222, p = 0.0344) and PP5-ERK2 (F(2,6) = 17.28, p = 0.0032) association in the presence of Rac1L61. Tukey post-tests are shown as follows: *, p < 0.05; **, p < 0.01. Data are mean ± S.E. No significant differences in the expression levels of FLAG-PP5, HA-ERK1, or HA-ERK2 were detected following normalization to HSP90 levels. C, HEK-293FT cells were transfected with pcDNA3 (Vector) or FLAG-PP5 in the absence (−) or presence (+) of constitutively active Rac1 (Rac1L61). Cells transfected with Rac1L61 were also treated with 100 ng/ml EGF for 5 min prior to lysis. Endogenous ERK1/2 immune complexes (ERK1/2 IPs) and cell lysates were analyzed by Western using phospho-ERK1/2 (p-ERK1/2), ERK2, PP5, and FLAG antibodies. D, HEK-293FT cells were co-transfected with HA-ERK2 and pcDNA3 (−) or Rac1L61 (+). Western analysis of cell lysates and proteins purifying with normal rabbit IgG (IgG IPs), rabbit anti-PP5 antibody (PP5 IPs), and microcystin-agarose (MC PDs) were performed using ERK2 and PP5 antibodies. E, lysates from untransfected (left) and HA-ERK2-expressing (right) HEK-293FT cells were incubated with microcystin-agarose, and bound proteins were extensively washed prior to splitting the resin into separate tubes, which were then incubated with buffer lacking (−) or containing (+; 20 μg) purified S100A1. A fraction of the reaction mixture was collected and analyzed by SDS-PAGE (15% Tris-glycine gels), and stained with Coomassie G-250 to detect S100A1. Following incubation, bound proteins were extensively washed and eluted for analysis by Western blotting with antibodies detecting the ERK1/2 and PP5 proteins. Unpaired, one-tailed t tests identified a significant decrease in the levels of bound ERK following incubation with S100A1 (left, *, p = 0.0117; right, *, p = 0.0279). Error bars, S.E. The data are representative of experiments performed three (A), three (C), two (D), three (E, left), and two (E, right) independent times with similar results.