FIGURE 3.
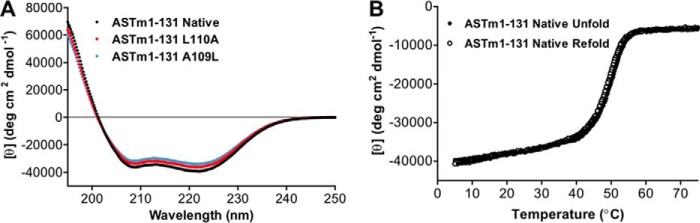
Folding and reversibility of Tm(1–131) wild type and mutant proteins using CD spectroscopy. A, CD spectrum scans of Tm(1–131) wild type (black), L110A (red), and A109L (light blue) measured immediately after thermal denaturation to 75 °C and cooling back to 5 °C. These scans indicate helical structure with very little difference in helical content between wild type and mutants. B, overlay of thermal denaturation or unfolding (dark circles) and refolding (open circles) profiles for wild type Tm(1–131) using CD. The profiles are shown overlapping, indicating equilibrium unfolding (reversibility of folding) in the temperature range of 5–75 °C. Tm(1–131) L110A and A109L both exhibited similar overlapping unfolding and refolding profiles. ASTm(1–131) indicates the presence of an N-terminal Ala-Ser dipeptide in these Tm(1–131) sequences. All profiles were measured with a temperature change of 1 °C/min.
