Abstract
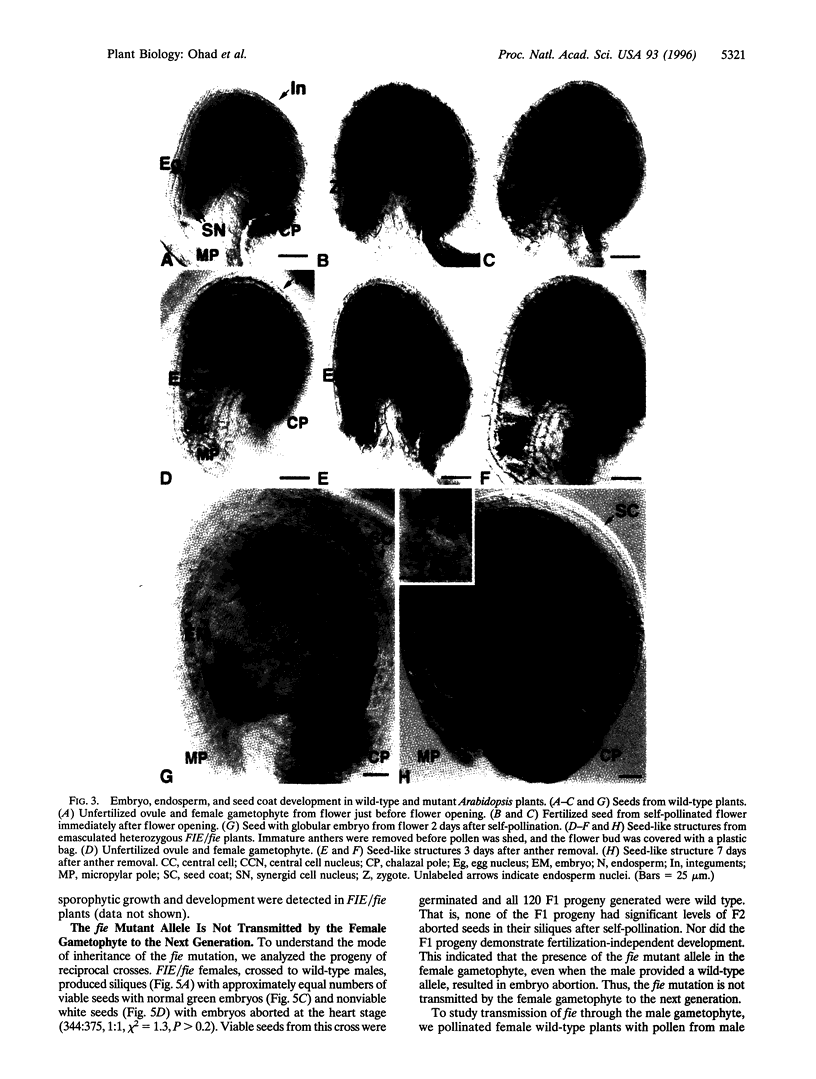
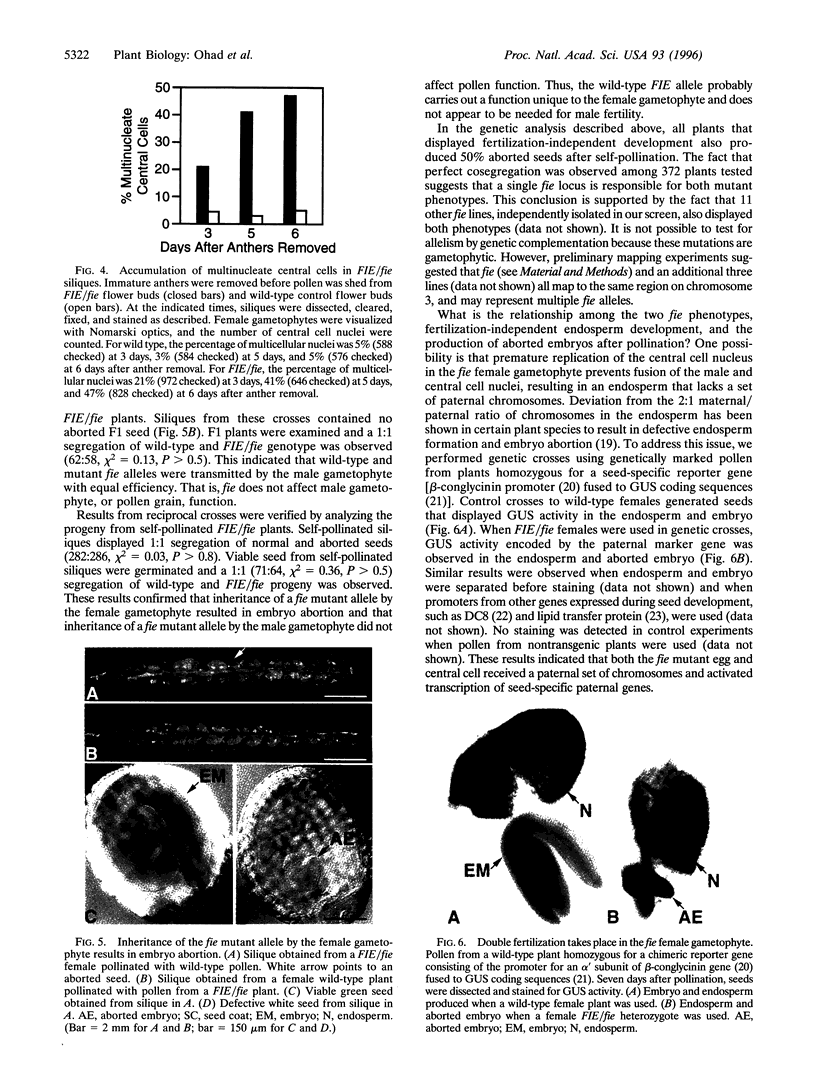
The mechanisms that initiate reproductive development after fertilization are not understood. Reproduction in higher plants is unique because it is initiated by two fertilization events in the haploid female gametophyte. One sperm nucleus fertilizes the egg to form the embryo. A second sperm nucleus fertilizes the central cell to form the endosperm, a unique tissue that supports the growth of the embryo. Fertilization also activates maternal tissue differentiation, the ovule integuments form the seed coat, and the ovary forms the fruit. To investigate mechanisms that initiate reproductive development, a female-gametophytic mutation termed fie (fertilization-independent endosperm) has been isolated in Arabidopsis. The fie mutation specifically affects the central cell, allowing for replication of the central cell nucleus and endosperm development without fertilization. The fie mutation does not appear to affect the egg cell, suggesting that the processes that control the initiation of embryogenesis and endosperm development are different. FIE/fie seed coat and fruit undergo fertilization-independent differentiation, which shows that the fie female gametophyte is the source of signals that activates sporophytic fruit and seed coat development. The mutant fie allele is not transmitted by the female gametophyte. Inheritance of the mutant fie allele by the female gametophyte results in embryo abortion, even when the pollen bears the wild-type FIE allele. Thus, FIE carries out a novel, essential function for female reproductive development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. J., Ecker J. R. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994 Jan 1;19(1):137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Castle L. A., Errampalli D., Atherton T. L., Franzmann L. H., Yoon E. S., Meinke D. W. Genetic and molecular characterization of embryonic mutants identified following seed transformation in Arabidopsis. Mol Gen Genet. 1993 Dec;241(5-6):504–514. doi: 10.1007/BF00279892. [DOI] [PubMed] [Google Scholar]
- Clark J. K., Sheridan W. F. Isolation and Characterization of 51 embryo-specific Mutations of Maize. Plant Cell. 1991 Sep;3(9):935–951. doi: 10.1105/tpc.3.9.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman W. E. Evidence of a pre-angiosperm origin of endosperm: implications for the evolution of flowering plants. Science. 1992 Jan 17;255(5042):336–339. doi: 10.1126/science.255.5042.336. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., de Paiva G., Yadegari R. Plant embryogenesis: zygote to seed. Science. 1994 Oct 28;266(5185):605–614. doi: 10.1126/science.266.5185.605. [DOI] [PubMed] [Google Scholar]
- Goupil P., Hatzopoulos P., Franz G., Hempel F. D., You R., Sung Z. R. Transcriptional regulation of a seed-specific carrot gene, DC8. Plant Mol Biol. 1992 Apr;18(6):1049–1063. doi: 10.1007/BF00047708. [DOI] [PubMed] [Google Scholar]
- Grafi G., Larkins B. A. Endoreduplication in maize endosperm: involvement of m phase--promoting factor inhibition and induction of s phase--related kinases. Science. 1995 Sep 1;269(5228):1262–1264. doi: 10.1126/science.269.5228.1262. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G. Genes to greens: embryonic pattern formation in plants. Science. 1992 Apr 24;256(5056):487–488. doi: 10.1126/science.256.5056.487. [DOI] [PubMed] [Google Scholar]
- Koltunow A. M. Apomixis: Embryo Sacs and Embryos Formed without Meiosis or Fertilization in Ovules. Plant Cell. 1993 Oct;5(10):1425–1437. doi: 10.1105/tpc.5.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A. M., Bicknell R. A., Chaudhury A. M. Apomixis: Molecular Strategies for the Generation of Genetically Identical Seeds without Fertilization. Plant Physiol. 1995 Aug;108(4):1345–1352. doi: 10.1104/pp.108.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Dellaert L. W., van der Veen J. H. EMS- and radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L.) Heynh. Mutat Res. 1982 Mar;93(1):109–123. doi: 10.1016/0027-5107(82)90129-4. [DOI] [PubMed] [Google Scholar]
- Lopes M. A., Larkins B. A. Endosperm origin, development, and function. Plant Cell. 1993 Oct;5(10):1383–1399. doi: 10.1105/tpc.5.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. E., Chourey P. S. The Maize Invertase-Deficient miniature-1 Seed Mutation Is Associated with Aberrant Pedicel and Endosperm Development. Plant Cell. 1992 Mar;4(3):297–305. doi: 10.1105/tpc.4.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T., Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 1991 Feb;7(2):45–49. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- Preuss D., Lemieux B., Yen G., Davis R. W. A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev. 1993 Jun;7(6):974–985. doi: 10.1101/gad.7.6.974. [DOI] [PubMed] [Google Scholar]
- Reiser L., Fischer R. L. The Ovule and the Embryo Sac. Plant Cell. 1993 Oct;5(10):1291–1301. doi: 10.1105/tpc.5.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. R., Bowman J. L., Meyerowitz E. M. Early flower development in Arabidopsis. Plant Cell. 1990 Aug;2(8):755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelly D. M., Peloquin S. J., Palmer R. G., Crane C. F. Mayer's hemalum-methyl salicylate: a stain-clearing technique for observations within whole ovules. Stain Technol. 1984 May;59(3):155–161. doi: 10.3109/10520298409113849. [DOI] [PubMed] [Google Scholar]
- Thoma S., Kaneko Y., Somerville C. A non-specific lipid transfer protein from Arabidopsis is a cell wall protein. Plant J. 1993 Mar;3(3):427–436. doi: 10.1046/j.1365-313x.1993.t01-25-00999.x. [DOI] [PubMed] [Google Scholar]