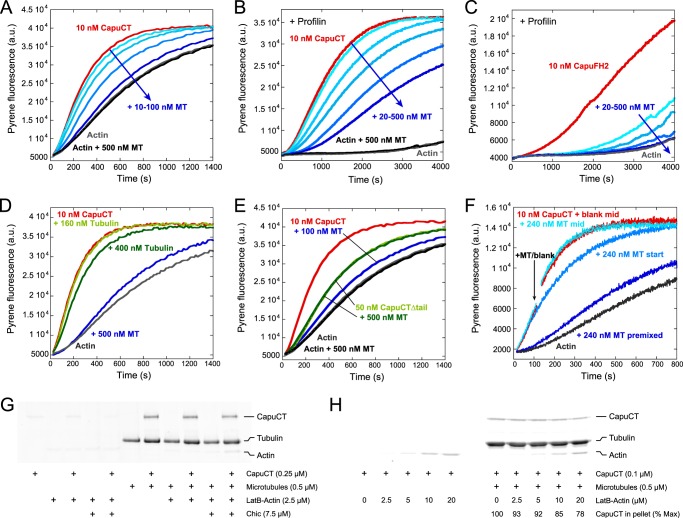
FIGURE 2.
Microtubules potently inhibit nucleation activity of Capu. A, dose-dependent inhibition of 10 nm CapuCT by 10, 20, 50, and 100 nm microtubules (MT) in bulk pyrene-actin polymerization assays. Microtubules do not affect baseline actin polymerization rates. B and C, microtubules inhibit nucleation from profilin-actin by 10 nm CapuCT (B; 20, 50, 100, 200, and 500 nm microtubules) and 10 nm CapuFH2 lacking the FH1 domain (C; 20, 50, 100, 500 nm microtubules). D, unassembled tubulin dimers have a negligible effect on CapuCT actin polymerization activity compared with taxol-stabilized microtubules. E, microtubules do not inhibit actin polymerization by CapuCTΔtail. At a 10:1 molar ratio, microtubules potently inhibit actin polymerization by CapuCT but not CapuCTΔtail. F, inhibition is sensitive to the time at which microtubules are added to the assay. Microtubules were added to CapuCT before adding actin (premixed), immediately after adding actin (start), or ∼120 s into the assay (mid, arrow indicates beginning of mixing; a.u., arbitrary units). G and H, LatB-stabilized actin monomers (LatB-Actin; 2-fold molar excess of LatB) weakly compete with microtubules to bind CapuCT in high-speed pelleting assays. Pellet fractions are shown, and concentrations are given. G, no noticeable competition was observed with 2.5 μm LatB-actin in the absence or presence of Chic (Drosophila profilin). H, some competition between microtubules and LatB-actin can be observed at very low CapuCT and very high LatB-actin concentrations, similar to those used in bulk pyrene-actin nucleation assays. CapuCT bound to microtubules is reported as a percentage of the maximum CapuCT bound at 0 μm LatB-actin.