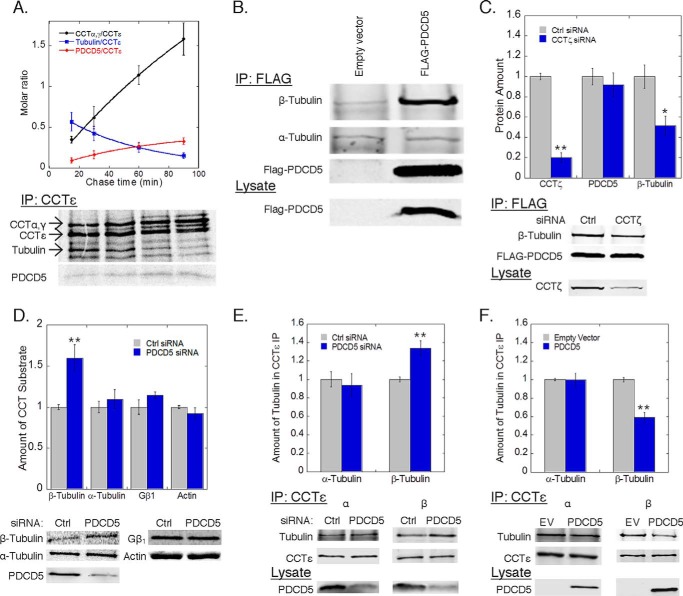
FIGURE 3.
PDCD5 inhibits β-tubulin folding. A, rate of association or dissociation from CCT complexes was measured by pulse-chase immunoprecipitations of CCTϵ from HEK-293T cells transfected with PDCD5-FLAG. The rate of association of CCTα and -γ subunits (black, t½ = 112 ± 18 min) and PDCD5 (red, t½= 44 ± 2 min) was calculated along with the rate of dissociation for tubulin (blue, t½ = 39 ± 1 min). B, binding of β-tubulin to PDCD5 was measured by co-immunoprecipitation from HEK-293T cells transfected with FLAG-PDCD5 or empty vector. C, effect of CCT knockdown on β-tubulin binding to PDCD5 was measured by co-immunoprecipitation from HEK-293T cells treated with CCTζ siRNA or a control siRNA and later transfected with FLAG-PDCD5. The ratio of the β-tubulin band to the PDCD5 band was calculated and normalized to the control. D, folding of the indicated proteins by CCT was measured by pulse-chase co-immunoprecipitations from HEK-293T cells treated with PDCD5 siRNA or negative control as indicated (see “Experimental Procedures”). E and F, effect of PDCD5 knockdown (E) or overexpression (F) on β-tubulin binding to CCT was measured by co-immunoprecipitation with CCTϵ and immunoblotting as indicated. The ratio of the β-tubulin band to the CCTϵ band was calculated and normalized to the control. In all experiments, bars represent the average ± S.E. from at least three experiments. Representative gels or blots are shown below each graph. PDCD5 knockdown averaged between 65 and 80% as measured by immunoblotting.