Figure 4.
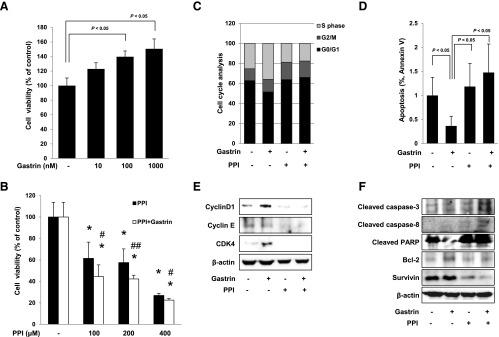
Changes of cell viability, cell cycle, and apoptosis in HCT116 cells. Cell viability was observed with MTT assay. (A) Gastrin significantly increased cell viability in a dose-dependent manner (P < .05). Conversely, PPI decreased cell viability in a dose-dependent manner (P < .05). (B) The combination of 100, 200, or 400 µM PPI and 100 nM gastrin paradoxically augmented significant decreases in cell viability (P < .05). All experiments were independently repeated at least three times. *P < .05 versus control; #P < .05 versus PPI; ##P < .01 versus PPI. (C) Flow cytometric analysis of HCT116 cells synchronized at the G1/S transition by serum starvation and released into the cell cycle with gastrin, PPI alone, or the combination of PPI and gastrin. The percentage of cells in different phases of the cell cycle was determined by using the Mod-Fit program. (D) HCT116 cells were treated with gastrin, PPI, or the combination for 12 hours and analyzed by flow cytometry for fluorescein isothiocyanate-annexin V and propidium iodide staining to verify the induction of apoptosis. The percentage of cells in apoptosis was determined by using the Mod-Fit program. (E) HCT116 cells were challenged with gastrin, PPI, or the combination of PPI and gastrin for 24 hours to investigate the expression of cell cycle markers. The expressions of cyclin D1, cyclin E, and CDK4 were assessed by Western blot analysis. (F) Western blot analysis was performed with antibodies of Bcl-2, cleaved caspase-3 and cleaved caspase-8, PARP, and survivin, respectively. PPI-treated cells led to apoptosis and cells with the combination of PPI and gastrin promoted more apoptosis than cells exposed to PPI alone.
