Abstract
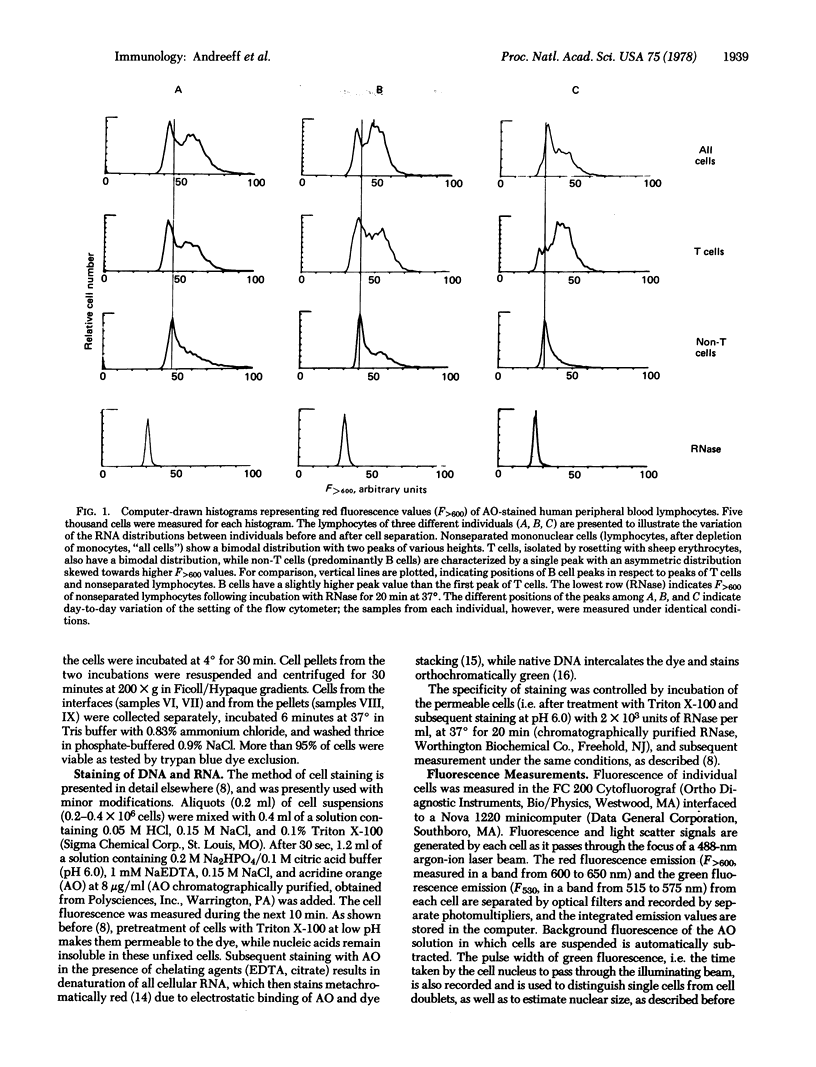
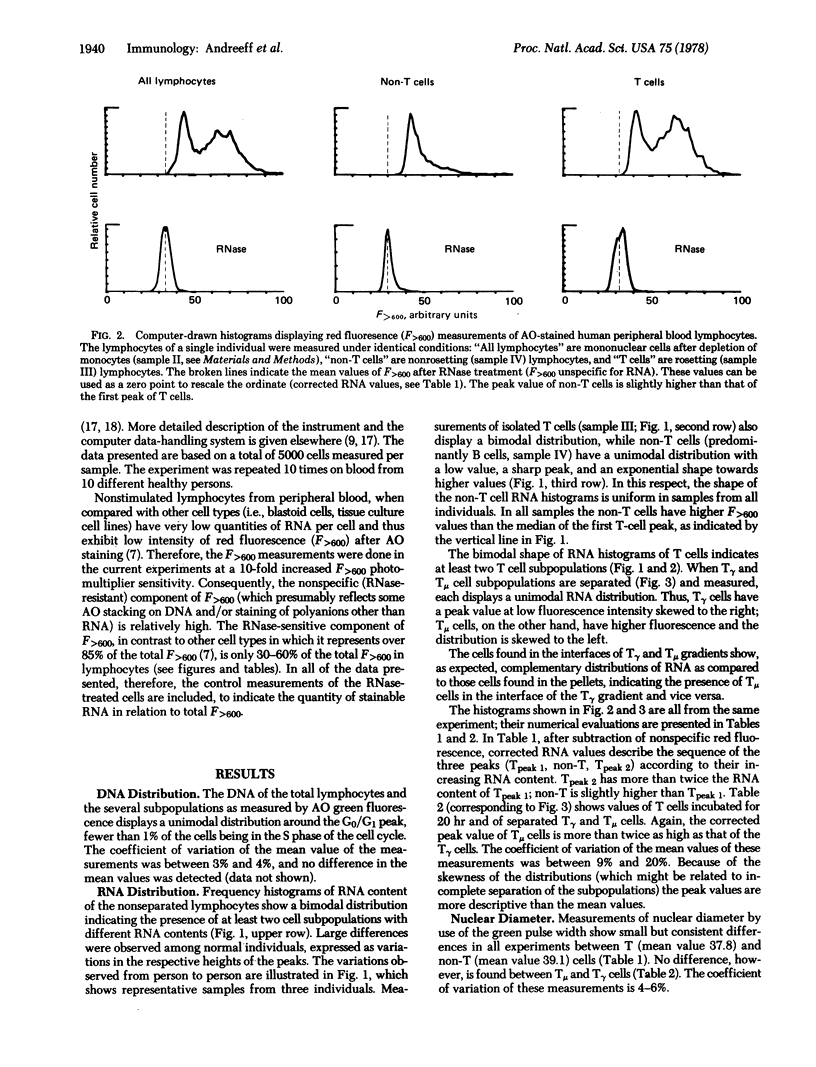
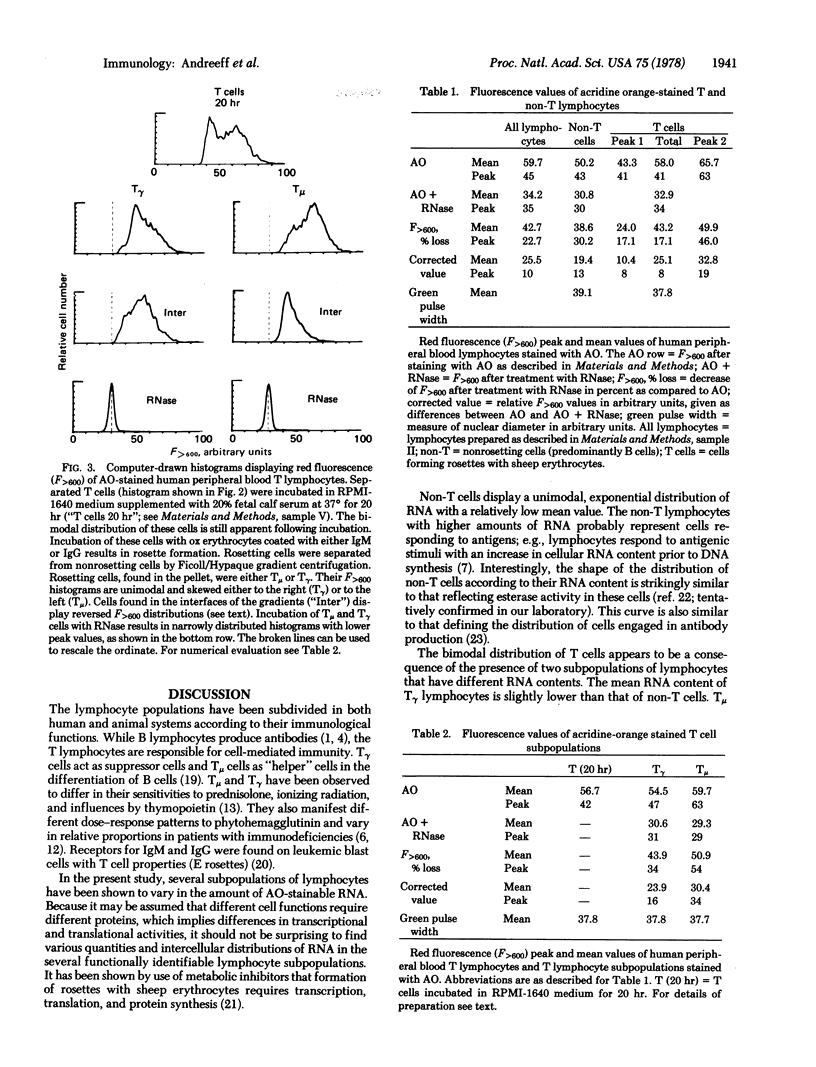
Human peripheral blood lymphocytes are stained with the metachromatic dye acridine orange and the fluorescence of individual cells is measured by flow cytometry. The relative content of stainable RNA per cell is estimated by comparison with RNase-treated cells. Non-T and T lymphocytes have different mean quantities of RNA per cell, and these classes exhibit different distributions of RNA content. Non-T cells have a unimodal distribution with a sharp peak and exponential distribution towards higher RNA values. T cells have a bimodal distribution with two separate peaks. When T cells having receptors for IgG (Tgamma cells) and IgM (Tmu cells) are separated, each of these cell populations displays a unimodal distribution. Of these three lymphocyte subpopulations, Tgamma cells have the lowest content of RNA per cell. Non-T cells have slightly higher RNA content than Tgamma, and Tmu cells have twice as much RNA as Tgamma cells. The RNA content, which surely relates to the different functions of these lymphocyte subpopulations, may also be a useful marker for rapidly distinguishing the lymphocyte subpopulations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentwich Z., Kunkel H. G. Specific properties of human B and T lymphocytes and alterations in disease. Transplant Rev. 1973;16:29–50. doi: 10.1111/j.1600-065x.1973.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology. 1977 Jun;10(2):71–76. [PubMed] [Google Scholar]
- Bradley D. F., Wolf M. K. AGGREGATION OF DYES BOUND TO POLYANIONS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):944–952. doi: 10.1073/pnas.45.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushkin S. C., Pantic V. S., Good R. A. RNA and protein synthesis in spontaneous rosette formation by T lymphocytes. J Immunol. 1975 Sep;115(3):866–870. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Conformation of RNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp Cell Res. 1975 Oct 1;95(1):143–153. doi: 10.1016/0014-4827(75)90619-9. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Cohnen G., Szaniawski W., Brittinger G. Scanning electron microscopic study of leukemic human B lymphocytes. Acta Haematol. 1977 Mar;57(4):247–256. doi: 10.1159/000207888. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. I. Studies in immunodeficient patients. Clin Exp Immunol. 1977 Nov;30(2):222–228. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. II. Effect of thymopoietin, corticosteroids, and irradiation. Cell Immunol. 1977 Nov;34(1):10–18. doi: 10.1016/0008-8749(77)90224-6. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken M. R., Parks D. R., Herzenberg L. A. Two-color immunofluorescence using a fluorescence-activated cell sorter. J Histochem Cytochem. 1977 Jul;25(7):899–907. doi: 10.1177/25.7.330738. [DOI] [PubMed] [Google Scholar]
- Melamed M. R., Darzynkiewicz Z., Traganos F., Sharpless T. Cytology automation by flow cytometry. Cancer Res. 1977 Aug;37(8 Pt 2):2806–2812. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Moretta A., Lydyard P. M. Receptors for IgM are expressed on acute lymphoblastic leukemic cells having T-cell characteristics. Clin Immunol Immunopathol. 1977 May;7(3):405–409. doi: 10.1016/0090-1229(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Sharpless T. K., Melamed M. R. Estimation of cell size from pulse shape in flow cytofluorometry. J Histochem Cytochem. 1976 Jan;24(1):257–264. doi: 10.1177/24.1.1254921. [DOI] [PubMed] [Google Scholar]
- Sharpless T., Traganos F., Darzynkiewicz Z., Melamed M. R. Flow cytofluorimetry: discrimination between single cells and cell aggregates by direct size measurements. Acta Cytol. 1975 Nov-Dec;19(6):577–581. [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Wilder M. E., Cram L. S. Differential fluorochromasia of human lymphocytes as measured by flow cytometry. J Histochem Cytochem. 1977 Jul;25(7):888–891. doi: 10.1177/25.7.70458. [DOI] [PubMed] [Google Scholar]


