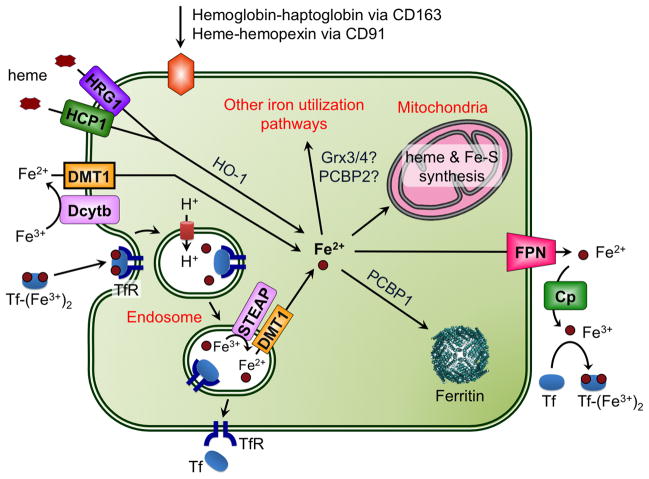
Figure 10.
Iron import and export in a generic mammalian cell. The plasma protein transferrin (Tf) binds 2 ferric ions for uptake by the transferrin receptors (TfR1 and TfR2). TfR1 is found in all cell types, while TfR2 is limited to liver, intestinal, and red blood cells. Tf(Fe3+)2-bound TfR is internalized by endocytosis and ferric iron is released in the acidic environment of the endosome. Tf and TfR are recycled to the plasma membrane. DMT1 is involved in release of Tf iron from the endosome following reduction of Fe3+ to Fe2+ by STEAP ferrireductases. Dcytb (cytochrome b-like ferrireductase) reduces dietary Fe3+ to Fe2+, which is imported by DMT1 at the plasma membrane. The plasma proteins haptoglobin and hemopexin bind hemoglobin and free heme, respectively, produced by erythrocyte destruction. Haptoglobin-hemoglobin and heme-hemopexin complexes are recognized by CD163 and CD91 receptors, respectively, for subsequent endocytosis. Heme is also imported via HCP1 and HRG1. HO-1 (heme oxygenase-1) catalyzes degradation of the heme to remove iron. Imported iron can be stored in ferritin or trafficked to the mitochondria for synthesis of heme and Fe-S clusters. FPN is the iron exporter, transporting Fe2+ out of the cell. Ceruloplasmin (Cp) oxidizes this Fe2+ to Fe3+ for binding to Tf.