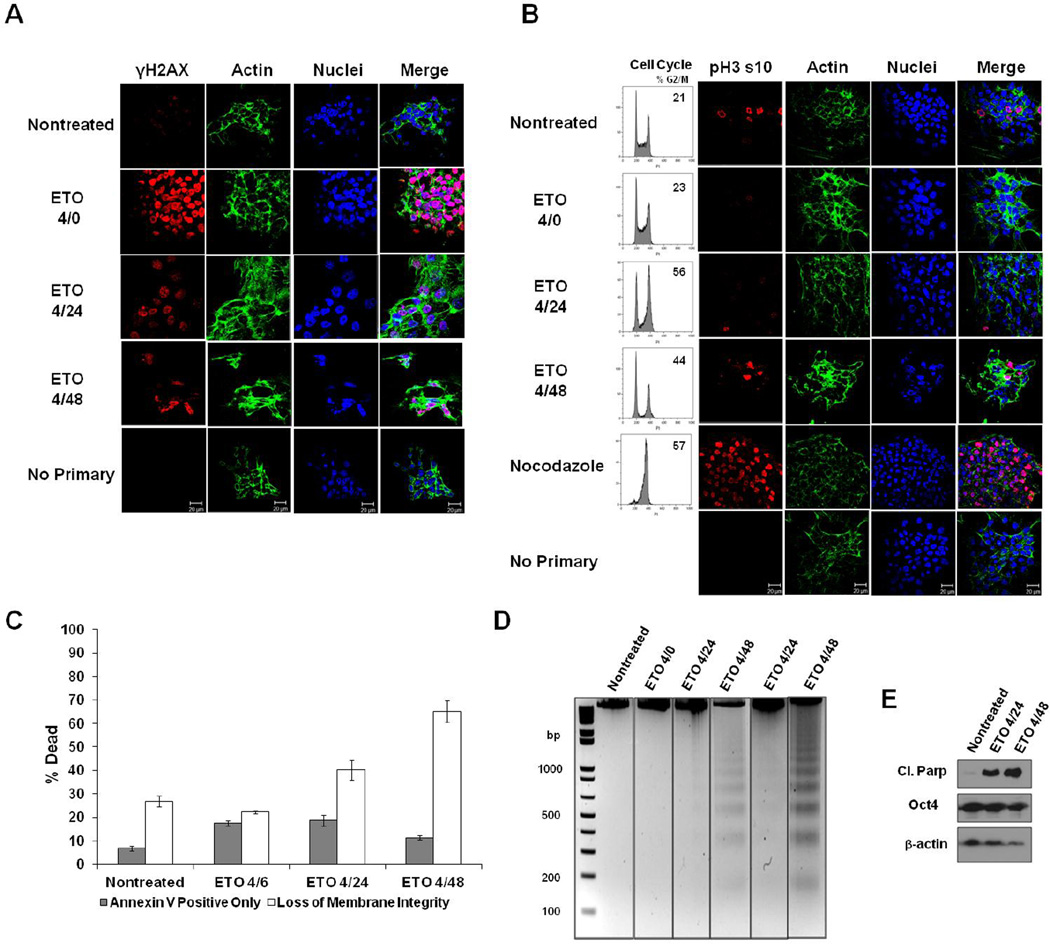
Figure 1. ESC responses to ETO.
A. ESCs were fixed immediately after 10µM etoposide (ETO) treatment (4/0-4 hour treatment; no recovery), or after 20 or 44 hours after treatment and recovery (4/24 or 4/48), or left nontreated. Cells were stained with antibody to γH2AX, and actin was stained with Alexa fluor 488-conjugated phalloidin. Nuclei were stained with Draq5. Cells stained with only secondary fluorescent antibody served as a control. B. Cells were treated as indicated and analyzed for their position in the cell cycle. To determine whether ETO induces a G2- or M-phase arrest, cells were stained with antibody to Histone H3 phospho-serine 10 (pH3 s10). Nocodazole-treated cells served as a control for mitotic arrest. C. ESCs were treated as indicated and death levels measured by flow cytometry after staining with Annexin V and a membrane impermeable dye. Data is the result of three trials. Error bars represent the standard error of the mean (SEM). D. DNA fragmentation assay on ESCs treated or left nontreated. DNA was isolated from all cells (floating and attached), and electrophoresed on 2% agarose gels. Two trials are displayed. E. Western blot using whole cell protein lysates from treated or nontreated ESCs. Expression of cleaved Parp-1 was monitored during the recovery period. Oct4 served as a marker for the level of ESC differentiation. Actin was used as a loading control.