Figure 1.
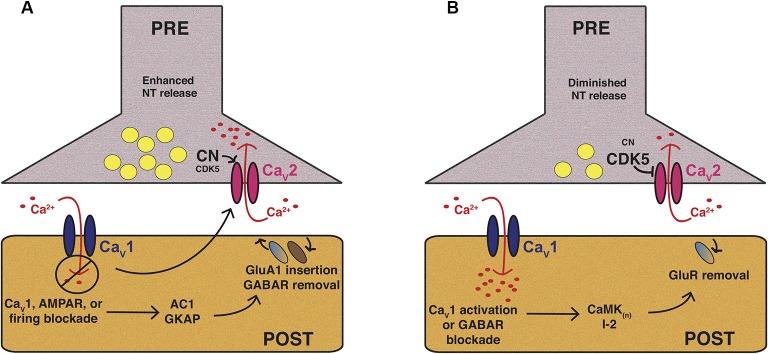
Mammalian CaV1 and CaV2 channels play central roles in forms of homeostatic plasticity. These two cartoons attempt to synthesize knowledge of mammalian pre- and postsynaptic homeostatic plasticity mechanisms involving CaV1 and CaV2 calcium channels in preparations like cultured hippocampal neurons. The cartoons are not intended to depict a single synaptic preparation or universally conserved mechanism, though some molecular responses may be widely conserved. (A) Homeostatic potentiation of synapse function. Inhibition of synaptic activity or postsynaptic CaV1 calcium influx results in multiple changes, including postsynaptic signaling through molecules like adenylate cyclase 1 (AC1) or guanylate kinase-associated protein (GKAP) to drive activating mechanisms, such as glutamate receptor insertion. Trans-synaptic signaling controlled by factors like Target of Rapamycin (TOR) and Brain-Derived Neurotrophic Factor (BDNF) can trigger enhanced presynaptic release probability. From a variety of systems there is evidence for enhanced presynaptic calcium influx through CaV2—which may require diminishment of cyclin-dependent kinase 5 (CDK5) function—as well as an enhanced readily releasable pool of presynaptic vesicles. (B) Homeostatic downscaling of synapse function. Synaptic activation (e.g., through GABA receptor blockade) and/or enhanced postsynaptic calcium influx through CaV1 results in the activation of diverse pathways, such as those mediated by calcium/calmodulin-dependent kinases (CaMK), as well as the Protein Phosphatase 1 (PP1) inhibitor, I-2. This can result in removal of excitatory glutamate receptors from the synapse. Presynaptically, there is evidence of diminished calcium influx through CaV2, and thus, diminished evoked presynaptic release.
