Abstract
Context
The cost-effectiveness of 13-valent pneumococcal conjugate vaccine (PCV13) compared with 23-valent pneumococcal polysaccharide vaccine (PPSV23) among US adults is unclear.
Objective
To estimate the cost-effectiveness of PCV13 vaccination strategies in adults to assist vaccination policy decision-making.
Design, Setting, and Population
A Markov state-transition model, lifetime time horizon, societal perspective. Simulations were performed in hypothetical cohorts of US 50-year-olds. Vaccination strategies and effectiveness estimates were developed by a Delphi expert panel; indirect (herd immunity) effects resulting from childhood PCV13 vaccination were extrapolated based on observed PCV7 effects. Data sources for model parameters included CDC Active Bacterial Core surveillance, National Hospital Discharge Survey and Nationwide Inpatient Sample data, and the National Health Interview Survey.
Main Outcome Measures
Pneumococcal disease cases prevented and incremental costs per quality-adjusted life year (QALY) gained.
Results
In the base case scenario, PCV13 given as a substitute for PPSV23 in current recommendations (i.e., vaccination at 65 years and at younger ages if comorbidities are present) cost $28,900/QALY gained compared with no vaccination and was more cost-effective than the currently recommended PPSV23 strategy. Routine PCV13 at ages 50 and 65 years cost $45,100/QALY compared with PCV13 substituted in current recommendations. Adding PPSV23 at age 75 to PCV13 at ages 50 and 65 years gained 0.00002 QALYs, costing $496,000/QALY gained. Results were robust in sensitivity analyses and alternative scenarios, except when low PCV13 effectiveness against nonbacteremic pneumococcal pneumonia was assumed or when greater childhood vaccination indirect effects were modeled. In these cases, PPSV23 as currently recommended was favored.
Conclusions
Overall, PCV13 vaccination was favored compared to PPSV23, but the analysis is sensitive to assumptions about PCV13 effectiveness against NPP and the magnitude of potential indirect effects from childhood PCV13 on pneumococcal serotype distribution.
Introduction
The 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been recommended for prevention of invasive pneumococcal disease (IPD) in adults since 19831. Most studies show that PPSV23 provides some protection against IPD, but studies have reached contradictory conclusions for its prevention of nonbacteremic pneumococcal pneumonia (NPP)1-2, which causes several hundred thousand illnesses annually in the US3. Large randomized controlled trials of PPSV23 conducted in developed countries have not found evidence of efficacy against NPP among community-dwelling older adults or among younger adults with chronic illness1, 4-5. Routine childhood vaccination with the 7-valent pneumococcal conjugate vaccine (PCV7) has dramatically decreased both IPD and NPP in children through both direct and indirect (herd immunity) vaccine effects6-7 and reduced adult pneumococcal disease through indirect effects6-9. The introduction of a pediatric conjugate vaccine containing six additional serotypes (PCV13) is expected to further reduce pneumococcal disease in children and adults10-11.
Prior analyses suggest that adult pneumococcal conjugate vaccination could prevent more disease than PPSV23, due to its potential effectiveness against both NPP and IPD12. Although PCV7 has been shown to prevent NPP in children13, PCV13 effectiveness in preventing NPP in adults is currently unknown and the subject of an ongoing clinical trial14. In addition, routine childhood vaccination with PCV13 will likely result in further indirect effects in adults10, perhaps limiting the potential benefits of adult vaccination. As PCV13 is currently under FDA licensure review for use among adults 50 years and older15, decisions about vaccination policy must weigh tradeoffs between the possibility of decreased NPP vs. fewer serotypes covered by PCV13, on a background of childhood vaccination-related changes in pneumococcal epidemiology and suboptimal adult vaccination uptake16. To address these issues, we used decision modeling techniques to examine the effectiveness and cost-effectiveness of pneumococcal vaccination strategies among adult cohorts 50 years of age and older.
Methods
Using a Markov state-transition model (Figures 1 and 2), we examined six pneumococcal vaccination strategies developed by a Delphi expert panel process (see below): 1) no vaccination, 2) the present US Advisory Committee on Immunization Practices (ACIP) adult recommendations (vaccinate all persons with PPSV23 at age 65; those who received PPSV23 before age 65 for a comorbid condition are recommended to receive another dose at age 65 or later if at least 5 years have passed since the previous dose)1, 3) substituting PCV13 for PPSV23 in current ACIP recommendations, 4) PCV13 at age 50 and PPSV23 at age 65, 5) PCV13 at ages 50 and 65, and 6) PCV13 at ages 50 and 65, then PPSV23 at age 75. Strategies were compared using identical hypothetical cohorts of 50 year old US adults, with cohorts followed as they aged. We used a lifetime time horizon, a societal perspective, and a 3% discount rate for costs and benefits, converting costs to 2006 US dollars17. Quality of life was modeled using health state utility weights, with 0 equaling death and 1 denoting perfect health; quality adjusted life years (QALY) are the product of the health state utility and the length of time in that state.
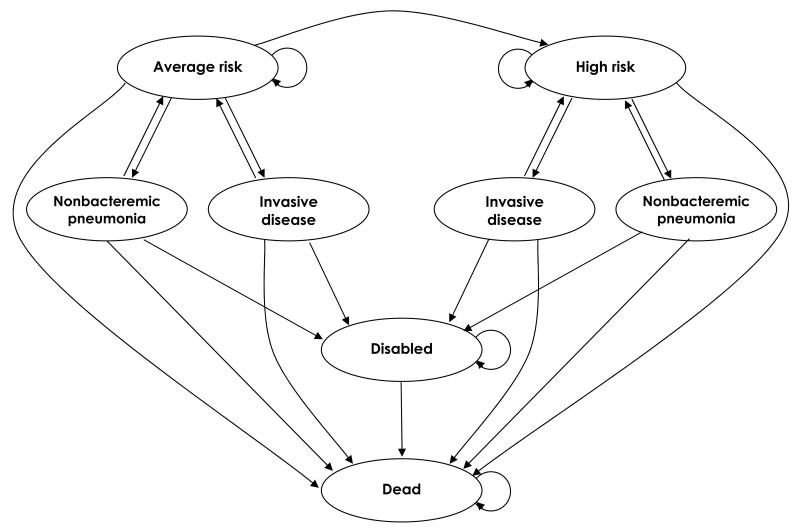
Figure 1. Schematic depiction of the Markov model for pneumococcal vaccination and infection.
Model health states are shown as ovals. During yearly model cycles, transitions between health states or remaining in the same health state can occur, represented by the arrows. Transitions to pneumococcal disease states are based on vaccination effects and herd immunity projections.
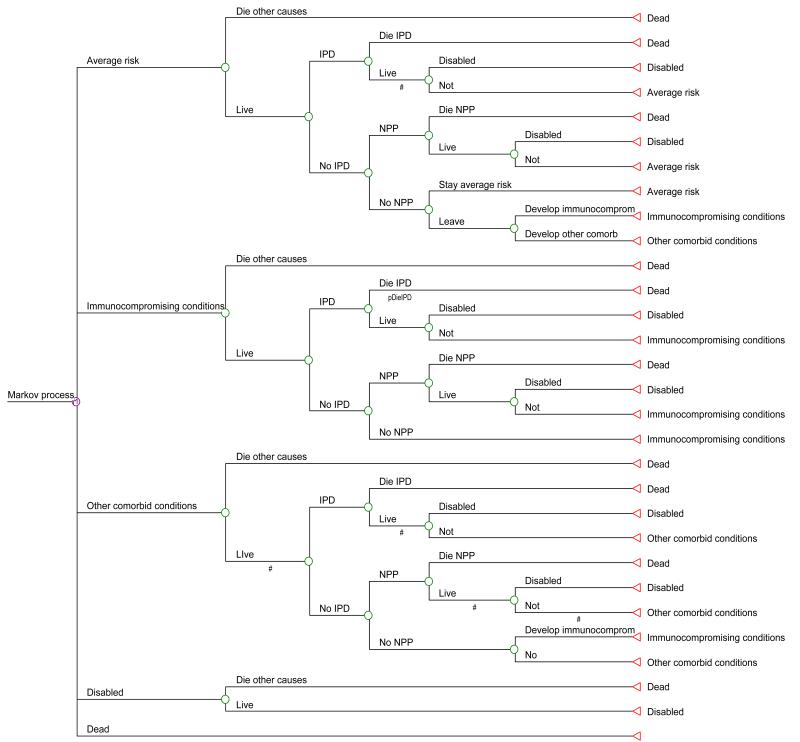
Figure 2. Markov cycle tree.
The cycle tree gives more detail on the programming of the Markov model. Health states are the first branches off the Markov node. Pneumococcal diseases (invasive pneumococcal disease, IPD and nonbacteremic pneumococcal pneumonia, NPP) are modeled as virtual states (“tolls”) within the tree structure. The triangular terminal nodes (on the left) depict the health states where portions of the cohort following that path will begin the next yearly cycle of the model.
The Markov model followed cohorts yearly until death. Pneumococcal disease was assumed to occur as in the Box, where the risk of infection is modified the likelihood and effectiveness of vaccination and by projected herd immunity effects from childhood PCV13. In the model, these factors varied by age and presence or absence of comorbidity. In the base case scenario, PPSV23 was assumed to have no effect on NPP4, while PCV13 was assumed to protect against NPP; these assumptions were modified in sensitivity analyses. Individuals could be in health states where pneumococcal disease risk was average or high and, within high-risk health states, persons with immunocompromising conditions were considered separately from those with other comorbid conditions. Persons at average risk could transition to the high-risk group due to development of a comorbid condition. We assumed no transitions from high risk to average risk. Patients could become disabled or die from pneumococcal infection, or recover.
We used 2006 National Health Interview Survey (NHIS) data to segment cohorts into comorbid illness groups to model differential vaccine effectiveness and pneumococcal disease rates based on age and comorbidity, using Centers for Disease Control and Prevention (CDC) definitions for immunocompromising and other comorbid conditions18. Asthma and cigarette smoking were recently added as pneumococcal vaccination indications1. In the model, asthma was included as a comorbid condition; smoking was not, due to difficulties in capturing reliable smoking information in CDC data. We also used Framingham Study19-22 and SEER23 data to model age-related risk of comorbid conditions in the oldest age groups. Patients with both immunocompromising and other comorbid conditions were included in the immunocompromised group; persons with HIV were also assumed to be included in the immunocompromised group. Transitions from other comorbid conditions to immunocompromised were based on SEER age-specific cancer incidence rates23.
CDC Active Bacterial Core surveillance (ABCs) data from 2007-2008 were used to model IPD rates and age-specific likelihood of illness from vaccine-contained serotypes (Table 1). We used previously published methods12 to derive hypothetical “no vaccine” IPD rates. Deaths were modeled using US mortality tables24. For hospitalized NPP rates, we extrapolated from all-cause pneumonia hospitalization rates (Table 2), estimating that pneumococcal pneumonia accounts for 30% of all-cause pneumonia hospitalizations3, 12, 25. We used National Hospital Discharge Survey data26 for our base case analysis, with Nationwide Inpatient Sample data6 used in sensitivity analyses. We assumed that the relative likelihood of hospitalized NPP among age- and comorbidity-specific groups was similar to that observed for IPD, and used rate ratios to model this assumption. Age- and comorbidity-specific rate ratios for IPD, from ABCs data, were applied to NPP rates to calculate age- and comorbidity-specific hospitalized NPP rates (Table 3). We also assumed that the serotype distributions were similar for hospitalized NPP and IPD. Due to uncertainty regarding the age- and comorbidity-specific frequency and serotype distribution of outpatient NPP and its lower cost27-28, we limited our analysis to hospitalized NPP.
Table 1. Characteristics of Invasive Pneumococcal Infections Based on the Active Bacterial Core Surveillance System*.
| Ages 50-59 | Age 60-69 | Age 70-79 | Age ≥ 80 | |
|---|---|---|---|---|
| Invasive pneumococcal disease rates (per
100,000 population per year) |
||||
| Total | 19.6 | 25.9 | 33.9 | 60.1 |
| No comorbid or immunocompromising conditions | 9.2 | 13.1 | 16.2 | 33.9 |
| ≥1 immunocompromising conditions | 67.1 | 58.5 | 54.1 | 64.3 |
| ≥1 comorbid conditions | 39.2 | 39.2 | 48.5 | 97.2 |
| PPSV23 vaccine serotype coverage (%) | 73.3% | 74.1% | 65.8% | 62.9% |
| PCV13 vaccine serotype coverage (%) | 48.3% | 48.7% | 40.8% | 40.8% |
| Disease outcome rates (per 100,000 population per year) | ||||
| Meningitis | 1.4 | 1.6 | 1.3 | 1.3 |
| Mortality | 2.1 | 2.9 | 3.9 | 11.9 |
Source: Active Bacterial Core Surveillance (ABCs) 2007-2008, Centers for Disease Control and Prevention, from all counties in ABCs PPSV23 = pneumococcal polysaccharide vaccine, PCV13 = 13-valent pneumococcal conjugate vaccine
Table 2. Annual US all-cause pneumonia hospitalization rates (per 100,000).
Table 3. Derived rates for hospitalized nonbacteremic pneumococcal pneumonia rates (per 100,000).
| Total | No conditions | Immunocompromised | Other comorbidities | |
|---|---|---|---|---|
| Ages 45-64 | 100.2 | 47.3 | 343.6 | 201 |
| Ages 65+ | 567 | 284.9 | 868 | 874.9 |
As described previously29, an expert panel, using the modified Delphi technique30, estimated PPSV23 effectiveness against IPD (Table 4). A second Delphi panel, comprised of ACIP Pneumococcal Vaccines Working Group members, estimated PCV13 effectiveness against vaccine serotypes causing IPD and NPP (Table 5) and selected the vaccination strategies to be modeled. PCV13 relative effectiveness against NPP was estimated to be 18% lower than for IPD (range 10-60%) in healthy 50 year olds, 25% lower (range 10-60%) in healthy 65 year olds, and 30% lower (10-60%) in persons with immunocompromising and/or other comorbid conditions; these calculations’ results are shown in Table 6. Disability after IPD was modeled using meningitis as a proxy, understanding that not all meningitis is disabling but other IPD syndromes can be. For disability after NPP31, we used 50% of the IPD value and varied it from 0-100% in sensitivity analyses. Vaccination adverse event data and quality of life utilities were literature-based32-34. Vaccine35, administration36, and average adverse event costs ($0.03 per dose34) came from published sources. IPD and NPP hospitalization costs were obtained from 2006 Healthcare Cost and Utilization Project data37.
Table 4. Expert Panel Estimates of PPSV23 Effectiveness (%) in Preventing Vaccine Serotype Invasive Pneumococcal Disease.
| Vaccinee age/health: | Healthy 50 year olds* |
Healthy 65 year olds* |
Healthy 80 year olds* |
Immunocompromised (all
ages) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base case | Range | Base case | Range | Base case | Range | Base case | Range | |||||
| Low | High | Low | High | Low | High | Low | High | |||||
| Years post vaccination | ||||||||||||
| 1 | 93 | 80 | 95 | 80 | 60 | 90 | 67 | 20 | 85 | 0 | 0 | 25.5 |
| 3 | 89 | 74 | 94.5 | 73 | 50 | 83 | 53 | 0 | 83.5 | 0 | 0 | 18 |
| 5 | 85 | 66 | 90 | 58 | 30.5 | 80 | 32 | 0 | 75 | 0 | 0 | 5 |
| 7 | 60 | 40 | 75 | 33 | 13 | 48 | 10 | 0 | 30 | 0 | 0 | 5 |
| 10 | 20 | 0 | 30 | 0 | 0 | 10 | 0 | 0 | 10 | 0 | 0 | 2.5 |
| 15 | 0 | 0 | 20 | 0 | 0 | 10 | 0 | 0 | 10 | 0 | 0 | 2.5 |
Patients with other comorbid conditions vaccinated at these ages had the same base case and high range values, low range was decreased 25% relative to listed values
Base case denotes values used in the primary analysis. Values were varied over the ranges shown in sensitivity analyses.
Table 5. Expert Panel Estimates of PCV13 Effectiveness (%) in Preventing Vaccine Serotype Invasive Pneumococcal Disease.
| Vaccinee age/health: | Healthy 50 year olds* |
Healthy 65 year olds* |
Immunocompromised (all
ages) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case | Range | Base case | Range | Base case | Range | ||||
| Low | High | Low | High | Low | High | ||||
| Years post vaccination | |||||||||
| 1 | 90 | 70 | 100 | 85 | 60 | 95 | 50 | 0 | 80 |
| 3 | 80 | 55 | 95 | 80 | 45 | 90 | 45 | 0 | 60 |
| 5 | 70 | 50 | 90 | 70 | 30 | 87 | 35 | 0 | 50 |
| 10 | 50 | 40 | 80 | 50 | 15 | 68 | 25 | 0 | 40 |
| 15 | 45 | 0 | 60 | 33 | 0 | 60 | 5 | 0 | 35 |
Patients with other comorbid conditions vaccinated at these ages had base case estimates decreased 15% relative to listed values.
Table 6. Expert Panel Estimates of PCV13 Effectiveness (%) in Preventing Vaccine-Serotype Nonbacteremic Pneumococcal Pneumonia.
| Vaccinee age/health: | Healthy 50 year olds* |
Healthy 65 year olds* |
Immunocompromised (all
ages) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case | Range | Base case | Range | Base case | Range | ||||
| Low | High | Low | High | Low | High | ||||
| Years post vaccination | |||||||||
| 1 | 74 | 28 | 90 | 64 | 24 | 86 | 35 | 0 | 72 |
| 3 | 66 | 22 | 86 | 60 | 18 | 81 | 32 | 0 | 54 |
| 5 | 57 | 20 | 81 | 53 | 12 | 78 | 25 | 0 | 45 |
| 10 | 41 | 16 | 72 | 38 | 6 | 61 | 18 | 0 | 36 |
| 15 | 37 | 0 | 54 | 25 | 0 | 54 | 4 | 0 | 32 |
Patients with other comorbid conditions vaccinated at these ages had base case estimates decreased 30% relative to listed values.
Potential indirect effects from childhood PCV13 were modeled in two steps. First, changes in adult IPD rates were extrapolated from indirect effects seen post-PVC79. We assumed that age-specific decreases in IPD would occur in commonly carried serotypes (3, 6A, 7F, and 19A) added to PCV13, as was observed for colonizing PCV7 serotypes9, and increases would occur in non-PCV13 serotype IPD. No herd effects were assumed for serotypes 1 and 5, which are considered uncommon colonizers11, 38. Next, age-specific changes in vaccine serotype distributions were modeled, based on observed changes after PCV7 introduction8, leading to a diminished relative likelihood of PCV13 serotype IPD in the first 1-5 years after PCV13 licensure. Longer-term effects were modeled based on observed age-related serotype distribution changes. Potential indirect PCV13 effects for NPP were modeled using point estimates for decreases observed after PCV7 introduction6.
In the base case scenario, we assumed 60.1% adherence to age-based vaccination recommendations and 33.9% adherence to comorbidity-based vaccination recommendations, based on observed PPSV23 uptake16, 39. In strategies modeling current vaccination recommendations, all persons with a comorbid condition diagnosed before age 50 were vaccinated at age 50. Persons aged 50 to 64 developing a comorbid condition were vaccinated that year, then revaccinated either at age 65 or five years after the first vaccination, whichever came last.
Table 7 depicts model parameter values. Parameters were varied individually in 1-way sensitivity analyses and varied simultaneously in probabilistic sensitivity analyses, where random draws from distributions were performed and the effectiveness or cost-effectiveness of each strategy calculated 3000 times. Distributions were chosen based on parameter characteristics and level of certainty. Parameters whose distributions were least certain (e.g., utility weights) were assigned uniform distributions, vaccine effectiveness estimates were assigned triangular distributions, and parameters whose distributions were most certain (i.e., derived from clinical trial or epidemiologic data) were assigned distributions based on data characteristics and ability to account for distribution skewness. In separate analyses, we varied the relative likelihood of IPD or NPP due to vaccine serotypes from 90-110% of the baseline values and considered greater herd immunity effects.
Table 7. Parameter Values and Ranges Examined in Sensitivity Analyses.
| Range | |||||
|---|---|---|---|---|---|
| Description | Base case | Low | High | Distribution | Source |
| Vaccine effectiveness (Delphi estimates by age/time) | |||||
| PPSV23 against IPD | Base | Low range | High range | Triangular | Expert panel (Table 4) |
| PCV13 against IPD | Base | Low range | High range | Triangular | Expert panel (Table 5) |
| PCV13 effectiveness against NPP relative to
IPD effectiveness |
Expert panel | ||||
| Age 50 | 82% of base | 40% | 90% | Beta | |
| Age 65 | 75% of base | 40% | 90% | Beta | |
| Immunocompromised | 70% of base | 40% | 90% | Beta | |
| Other comorbid conditions | 70% of base | 40% | 90% | Beta | |
| PPSV23 effectiveness against NPP | 0% | 0% | 80% | NA | |
| Relative risk of disease from vaccine serotypes | 1 | 0.9 | 1.1 | Uniform | Table 1 |
| Vaccine adverse events | |||||
| Duration of symptoms (days) | 3 | 1 | 8 | Exponential | 32 |
| Probability per vaccinee after first vaccination | 3.2% | 2.2% | 4.6% | Beta | 32 |
| Relative risk after subsequent vaccinations | 3.3 | 2.1 | 5.1 | Log normal | 32 |
| Disability | |||||
| Excess mortality per year | 0.1 | 0 | 1 | Uniform | Estimated |
| Risk relative to base case risk | 1 | 0.5 | 1.5 | Uniform | Table 1 * |
| Risk in nonbacteremic pneumonia relative to IPD | 0.5 | 0 | 1 | Uniform | Estimated |
| Case-fatality odds ratio for patients
with immunocompromising or other comorbid conditions |
1.5 | 1.3 | 1.8 | Log normal | 8 |
| Utility weights | |||||
| Average risk population | Uniform | 34 | |||
| 50-55 y | 0.83 | 0.78 | 0.88 | ||
| 55-60 y | 0.81 | 0.76 | 0.86 | ||
| 60-65 y | 0.77 | 0.72 | 0.82 | ||
| 65-70 y | 0.76 | 0.71 | 0.81 | ||
| 70-75 y | 0.74 | 0.69 | 0.79 | ||
| 75-80 y | 0.70 | 0.65 | 0.75 | ||
| 80-85 y | 0.63 | 0.58 | 0.68 | ||
| >85 y | 0.51 | 0.46 | 0.56 | ||
| High risk population | Uniform | 34 | |||
| 50-55 y | 0.72 | 0.67 | 0.77 | ||
| 55-60 y | 0.69 | 0.64 | 0.74 | ||
| 60-65 y | 0.63 | 0.58 | 0.68 | ||
| 65-70 y | 0.57 | 0.52 | 0.62 | ||
| 70-75 y | 0.54 | 0.49 | 0.59 | ||
| 75-80 y | 0.52 | 0.47 | 0.57 | ||
| 80-85 y | 0.51 | 0.46 | 0.56 | ||
| > 85 y | 0.51 | 0.46 | 0.56 | ||
| Invasive pneumococcal disease | 0.2 | 0.1 | 0.5 | Uniform | 34 |
| Hospitalized nonbacteremic pneumonia | 0.2 | 0.1 | 0.5 | Uniform | Estimate34 |
| Disabled | 0.4 | 0.2 | 0.6 | Uniform | Estimate33 |
| Vaccine adverse event | 0.9 | 0.8 | 0.99 | Uniform | Estimate33 |
| Costs | |||||
| Vaccine and administration | |||||
| PPSV23 | $43 | $25 | $67 | Gamma | 35-36 |
| PCV13 | $128 | $73 | $196 | Gamma | 35-36 |
| Invasive pneumococcal disease | |||||
| Discharged alive | |||||
| 50-64 | $24,519 | base | base | 37 | |
| 65-74 | $20,416 | base | base | 37 | |
| >74 | $17,166 | base | base | 37 | |
| Died | |||||
| 50-64 | $33,778 | base | base | 37 | |
| 65-74 | $29,263 | base | base | 37 | |
| >74 | $20,750 | base | base | 37 | |
| Nonbacteremic pneumonia | |||||
| Discharged alive | |||||
| 50-64 | $19,396 | base | base | 37 | |
| 65-74 | $16,925 | base | base | 37 | |
| >74 | $13,258 | base | base | 37 | |
| Died | |||||
| 50-64 | $35,408 | base | base | 37 | |
| 65-74 | $28,288 | base | base | 37 | |
| >74 | $21,560 | base | base | 37 |
Using meningitis rates as a proxy for disability incidence
PPSV23 = pneumococcal polysaccharide vaccine, PCV= pneumococcal conjugate vaccine, IPD = invasive pneumococcal disease, NPP = nonbacteremic pneumococcal pneumonia
Results
Pneumococcal disease epidemiology
With no vaccination, the lifetime risk from age 50 onward for hospitalized NPP was 9.3%, for IPD was 0.86%, and for death due to pneumococcal disease was 1.8%. Thus, among the cohort of approximately 4.3 million US 50-year-olds in 200640, the model estimated 396,087 NPP hospitalizations, 36,576 IPD cases and 75,647 deaths due to pneumococcal disease over their lifetime. Table 8, top, summarizes the public health impact of different vaccination strategies. PPSV23 strategies prevented more IPD than strategies using only PCV13, while strategies using 2 scheduled PCV13 doses prevented more NPP.
Table 8. Public health impact of various adult pneumococcal vaccination strategies in Markov models. Lifetime incidence of pneumococcal disease in 50-year-old cohorts.
| Base case analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospitalized
nonbacteremic pneumococcal pneumonia |
Invasive pneumococcal disease | Pneumococcal disease deaths | |||||||
| Incidence/105 | RR | Cases prevented* |
Incidence/105 | RR | Cases prevented* |
Incidence/105 | RR | Deaths prevented* |
|
| No vaccination | 9292 | 1.0 | - | 858 | 1.0 | - | 1775 | 1.0 | - |
| 65 and HR younger (PPSV23) | 9292 | 1.0 | 0 | 815 | 0.95 | 1834 | 1769 | 0.997 | 255 |
| PCV13 at 50, PPSV23 at 65 | 9229 | 0.99 | 2648 | 822 | 0.96 | 1522 | 1762 | 0.993 | 523 |
| 65 and HR younger (PCV13) | 9122 | 0.98 | 7217 | 833 | 0.97 | 1082 | 1749 | 0.985 | 1111 |
| PCV13 at 50 65 | 8988 | 0.97 | 12944 | 830 | 0.97 | 1207 | 1729 | 0.974 | 1947 |
| PCV13 at 50 65, PPSV23 at 75 | 8988 | 0.97 | 12944 | 822 | 0.96 | 1537 | 1728 | 0.974 | 1998 |
| Low PCV13 effectiveness against nonbacteremic pneumococcal pneumonia * | |||||||||
|
Hospitalized nonbacteremic
pneumococcal pneumonia |
Invasive pneumococcal disease | Pneumococcal disease deaths | |||||||
| Incidence/105 | RR |
Cases
prevented * |
Incidence/105 | RR |
Cases
prevented * |
Incidence/105 | RR |
Deaths
prevented * |
|
| No vaccination | 9292 | 1.0 | - | 858 | 1.0 | - | 1775 | 1.0 | - |
| 65 and HR younger (PPSV23) | 9292 | 1.0 | 0 | 815 | 0.95 | 1834 | 1769 | 0.997 | 255 |
| PCV13 at 50, PPSV23 at 65 | 9271 | 1.00 | 862 | 822 | 0.96 | 1522 | 1768 | 0.996 | 301 |
| 65 and HR younger (PCV13) | 9224 | 0.99 | 2867 | 833 | 0.97 | 1082 | 1761 | 0.993 | 559 |
| PCV13 at 50 65 | 9210 | 0.99 | 3489 | 830 | 0.97 | 1207 | 1760 | 0.992 | 632 |
| PCV13 at 50 65, PPSV23 at 75 | 9210 | 0.99 | 3489 | 822 | 0.96 | 1537 | 1759 | 0.991 | 683 |
RR = relative risk, HR = high risk, PPSV23 = 23-valent pneumococcal polysaccharide vaccine, PCV13 = 13-valent pneumococcal conjugate vaccine, NA = not applicable, not included in probabilistic sensitivity analysis
PCV effectiveness against nonbacteremic pneumonia set at the low range estimates of the Delphi expert panel
In sensitivity analyses, results were most sensitive to variation in vaccine effectiveness estimates and magnitude of herd effects from childhood PCV13 vaccination on adult NPP. Using Nationwide Inpatient Sample data for all-cause pneumonia rates instead of the National Hospital Discharge Survey minimally affected results.
Due to the unknown effectiveness of adult PCV13 against NPP, we also performed a worst-case NPP scenario (Table 8, bottom), where PCV13 effectiveness against NPP was set at the low range of estimates (Table 6) and base case IPD effectiveness estimates for both vaccines were used. Despite worst-case assumptions, more total pneumococcal disease cases and deaths were prevented by strategies containing PCV13 compared to the current PPSV23 recommendation strategy.
Cost-effectiveness analysis
Incremental cost-effectiveness analysis results under base case assumptions are shown in Table 9 (top). In accordance with guidelines17, we present results as incremental cost-effectiveness ratios, ordering strategies by cost and eliminating strategies that are strictly dominated (more costly and less effective) or extended dominated (having higher incremental cost-effectiveness ratios than more effective strategies)41.
Table 9. Cost-effectiveness analysis results.
| Cost | Incremental Cost |
Effectiveness (QALY) |
Incremental Effectiveness (QALY) |
ICER ($/QALY) |
|
|---|---|---|---|---|---|
| Base case | |||||
| No vaccination | $1,047 | - | 12.58345 | - | - |
| 65 and HR younger (PPSV23)* | $1,059 | $12 | 12.58380 | 0.00035 | (Ext Dom) |
| 65 and HR younger (PCV13) | $1,080 | $33 | 12.58461 | 0.00116 | $28,900 |
| PCV13 at 50, PPSV23 at 65 | $1,119 | $39 | 12.58449 | −0.00012 | (Dominated) |
| PCV13 at 50 65 | $1,123 | $43 | 12.58555 | 0.00094 | $45,100 |
| PCV13 at 50 65, PPSV23 at 75 | $1,131 | $8 | 12.58557 | 0.00002 | $496,000 |
| Worst case † | |||||
| No vaccination | $1,047 | - | 12.58345 | - | - |
| 65 and HR younger (PPSV23)* | $1,059 | $12 | 12.58380 | 0.00035 | $34,616 |
| 65 and HR younger (PCV13) | $1,092 | $34 | 12.58405 | 0.00027 | $131,486 |
| PCV13 at 50, PPSV23 at 65 | $1,127 | $35 | 12.58402 | -0.00003 | (Dominated) |
| PCV13 at 50 65 | $1,150 | $58 | 12.58428 | 0.00023 | $255,285 |
| PCV13 at 50 65, PPSV23 at 75 | $1,158 | $8 | 12.58430 | 0.00002 | $496,538 |
The currently recommended adult pneumococcal vaccination strategy
Lowest PCV13 effectiveness estimates against nonbacteremic pneumococcal pneumonia
PCV13 = 13-valent pneumococcal conjugate vaccination; PPSV = 23-valent pneumococcal polysaccharide vaccine; QALY = quality adjusted life year; ICER = incremental cost-effectiveness ratio; HR=high risk conditions
Ext Dom = extended dominance (other strategies have lower cost-effectiveness ratios than this strategy) Dominated = other strategies are less costly and more effective than this strategy. Based on recommendations, strategies that are dominated by either mechanism are eliminated from further consideration in a cost-effectiveness analysis.
With no vaccination, the total per person cost of IPD and hospitalized NPP from age 50 onward was $1047. Compared to no vaccination, current PPSV23 recommendations (vaccination at age 65 and at younger ages if comorbidities are present) cost $34,600/QALY gained. However, this strategy is eliminated due to extended dominance, since its cost-effectiveness ratio is greater than that of the more effective PCV13 substituted in current recommendations strategy, which cost $28,900/QALY gained. PCV13 given routinely at ages 50 and 65 years cost $45,100/QALY compared with PCV13 substituted in current recommendations. PCV13 at ages 50 and 65 followed by PPSV23 at age 75 gained 0.00002 more QALYs per person and cost $496,000/QALY gained. There are no absolute criteria for cost-effectiveness but, in general, interventions costing <$20,000/QALY gained are felt to have strong evidence for adoption, interventions costing $20,000-$100,000/QALY have moderate evidence, and those costing >$100,000/QALY have weaker evidence for adoption42-43.
When PCV13 effectiveness against NPP is set at low range estimates (Table 9, bottom), current PPSV23 recommendations were favored, costing $34,600/QALY gained. PCV13 substituted in current recommendations cost $131,000/QALY gained; other strategies had prohibitive cost-effectiveness ratios. If, in this scenario, PPSV23 effectiveness against IPD is also at the low estimates, the current PPSV23 recommendation strategy cost $60,200/QALY and PCV13 substituted in current recommendations cost $93,000/QALY gained.
The analysis was also sensitive to vaccine costs. PCV13 substituted into current recommendations cost >$100,000/QALY when PCV13 vaccination cost was >$237 (base case estimate=$128). The analysis was not sensitive to individual variation of other parameter values (Table 10).
Table 10. One-way sensitivity analysis: parameters whose variation results in ≥5% changes in incremental cost effectiveness ratios.
| Incremental cost-effectiveness ratio (per QALY) | ||||
|---|---|---|---|---|
| Base case value | Range values | PCV13 at 65 and HR younger | PCV13 at 50 65 | |
| Base case | - | - | $28,900 | $45,100 |
| Vaccine effectiveness estimates | Tables 4 & 5 | Low range | $46,400 | $175,000 |
| High range | $5,300 | $76,700 | ||
| PCV13 relative effectiveness against NPP | ||||
| Age 50 | 82% of base | 40% | $28,900 | $60,800 |
| 90% | $28,900 | $40,100 | ||
| Age 65 | 75% of base | 40% | $43,600 | $45,100 |
| 90% | $25,100 | $45,100 | ||
| Other comorbid conditions | 70% of base | 40% | $45,200 | $64,300 |
| 90% | $23,100 | $36,300 | ||
| RR of infection from vaccine serotypes | 1 | 0.9 | $33,000 | $50,300 |
| 1.1 | $25,600 | $40,800 | ||
| Disability risk in NPP relative to IPD | 0.5 | 0 | $34,000 | $53,000 |
| 1 | $24,800 | $38,900 | ||
| Case-fatality OR with immunocompromise
or other comorbid conditions |
1.5 | 1.3 | $30,600 | $47,300 |
| 1.8 | $26,700 | $42,300 | ||
| Vaccine and administration costs | ||||
| PPSV23 | $43 | $25 | $36,700 | $45,100 |
| $67 | $28,900 | $45,100 | ||
| PCV13 | $128 | $73 | $6,930 | $16,900 |
| $196 | $65,100 | $79,900 | ||
QALY = quality adjusted life-year, PCV13 = 13-valent pneumococcal conjugate vaccine, PPSV23 = 23-valent pneumococcal polysaccharide vaccine, NPP = noninvasive pneumococcal pneumonia, IPD = invasive pneumococcal disease, RR = relative risk
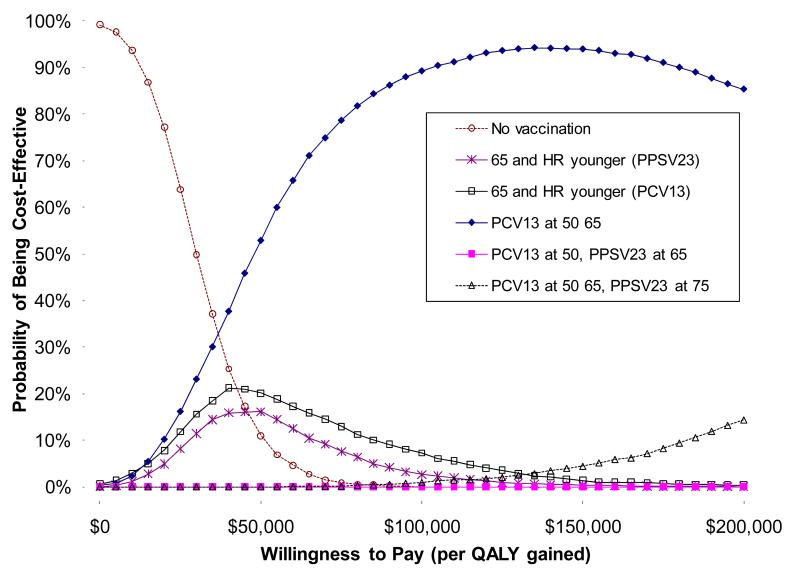
A probabilistic sensitivity analysis of base case results is shown as a cost-effectiveness acceptability curve (Figure 3), showing the proportion of cost-effectiveness calculations that would be considered acceptable from a societal standpoint for various willingness-to-pay thresholds. This analysis, which includes the likelihood of worst-case scenario for effectiveness against NPP, PCV13 given routinely at ages 50 and 65 would be favored if willingness-to-pay (or acceptability) thresholds were >$50,000/QALY. Changing vaccine effectiveness distributions from triangular to uniform or beta distributions left results essentially unchanged.
Figure 3. Probabilistic sensitivity analysis for adult pneumococcal vaccination strategies.
Results are shown as a cost-effectiveness acceptability curve. The y-axis shows the likelihood that strategies would be considered cost-effective for a given cost-effectiveness willingness to pay (or acceptability) threshold.
In a series of separate sensitivity analyses on the base case model, we relaxed modeling assumptions to test model robustness. If PCV13 had no direct effects on adult nonbacteremic pneumonia, the current PPSV23 policy cost $34,600/QALY and all PCV13 strategies were strictly dominated (i.e., more costly and less effective). If greater herd immunity effects on pneumococcal disease rates (leading to fewer IPD and NPP cases) were modeled, current recommendations using PCV13 cost $38,400/QALY and PCV13 at ages 50 and 65 cost $51,000/QALY gained. Modeling greater decreases in disease likelihood from a vaccine serotype (declining with age from a 16% relative likelihood of disease from PCV13 serotypes compared to nonvaccine serotypes at age 50, rather than from 24% in the base case) led to the current recommendations using PCV13 strategy being dominated, current recommendations using PPSV23 costing $38,900/QALY gained, and PCV13 at ages 50 and 65 costing $106,000/QALY. In the base case analysis, we assumed that 30% of hospitalized all-cause pneumonia was NPP. If NPP accounted for 20% pneumonia hospitalizations, current PPSV23 recommendations cost $34,400/QALY, PCV13 in current recommendations cost $59,100/QALY, and PCV13 at ages 50 and 65 cost $74,200/QALY.
We also examined scenarios where PPSV23 was effective against NPP. PCV13 at 50 and 65 would continue to be favored (at a $100,000/QALY criterion) if PPSV23 effectiveness against NPP was <44% at age 50 and <38% at age 65. At higher effectiveness levels, the currently recommended PPSV23 strategy was favored.
Discussion
Our analysis favors vaccinating adults with PCV13 instead of PPSV23, and suggests that PCV13 given either as a substitute for PPSV23 in current recommendations or routinely at ages 50 and 65 might reduce pneumococcal disease burden in an economically reasonable fashion. A two-dose PCV13 strategy at 50 and 65, although having a higher cost-effectiveness ratio, addresses the complexity of risk-based recommendations and is consistent with moves away from comorbidity-based strategies, exemplified by recent changes in influenza vaccination recommendations44.
However, results favoring PCV13 were sensitive to assumptions regarding PCV13 effectiveness against NPP. If effectiveness against NPP is low, then current PPSV23 recommendations are favored, but if, in addition, PPSV23 effectiveness against IPD is at the experts’ low range estimate, PCV13 substituted into current recommendations again becomes economically reasonable. This analysis highlights the tradeoffs that policy makers must consider when choosing among adult pneumococcal vaccination strategies. PPSV23 covers 23 serotypes and could prevent more IPD than PCV13 but, based on US and Western European studies, has no consistent effect on NPP4-5. Based on the experience with PCV7 program6-7, PCV13 is likely to prevent NPP. Since NPP is much more common than IPD, PCV13, despite its narrower serotype coverage, should prevent more pneumococcal disease than PPSV23. However, when modeling the possibility of PPSV23 preventing NPP, PPSV23 could be favored, but only if its effectiveness against NPP is higher than would be expected based on trial results4-5. At present, PCV13 effectiveness against pneumonia in adults is unknown. However, PCV7 was consistently effective against pediatric pneumonia in randomized clinical trials13, 46-48.
Results were also sensitive to the magnitude of indirect effects from childhood PCV13. Herd immunity from the added 6 serotypes will likely reduce disease rates in adults. Indirect effects of childhood PCV7 on carriage and IPD rates among adults have been documented8-9, 49. Decreases in all-cause pneumonia hospitalizations in adults 50 years and older were observed following PCV7 introduction, although these reductions were not statistically significant in one study6, but were significant in another7. However, given documented decreases in IPD rates due to indirect effects, we modeled decreases in adult NPP rates based on published point estimates, which could bias the analysis against PCV13. In any case, larger than expected indirect effects from childhood PCV13 would reduce the value of adult PCV13 strategies.
Several limitations should be considered in interpreting our findings. The analysis is limited by the lack of data on PCV13 effectiveness. A randomized trial of PCV13 among 85,000 adults ≥65 years of age is currently underway in the Netherlands; data collection will end 12/2011 but results will not be available until 201314. The magnitude of herd effects and changes in serotype distribution resulting from childhood PCV13 are also unknown. We modeled these based on data from the PCV7 experience. We excluded outpatient pneumonia due to difficulties in accurate estimation of disease rates and characteristics, and the relatively minor role of outpatient costs; again possibly biasing against PCV13. Other studies estimated that outpatient NPP accounted for ≤5% of adult pneumococcal disease costs28, 50. Our model is based on 50-year-old cohorts followed over their lifetime; this analysis does not consider cohorts of differing ages or other issues germane to ensuring population immunity, such as catch up vaccination. Finally, as infrastructure and resources to support adult vaccination are limited, achieving sufficient coverage with PCV13 is uncertain39, 51.
In conclusion, our analysis suggests that PCV13 might prevent more pneumococcal disease compared to the current PPSV23 vaccination recommendations, while remaining economically reasonable. However, these conclusions are sensitive to assumptions regarding PCV13 effectiveness against NPP, PPSV23 effectiveness against IPD and NPP, and herd immunity effects. Model estimates of adult PCV13 impact would be strengthened by evidence of PCV13 effectiveness against NPP from ongoing clinical trials and availability of data on the indirect effects of childhood PCV13 on adult pneumococcal disease rates.
Box.
Baseline probability of infection (pre-childhood PCV13) × Projected relative likelihood of infection (indirect effect, post- vs. pre-PCV13) × Relative likelihood of infection from a vaccine serotype (pre-PCV13) × Projected change in infection serotype likelihood (indirect effect) × Probability of being vaccinated × Probability of vaccine effectiveness
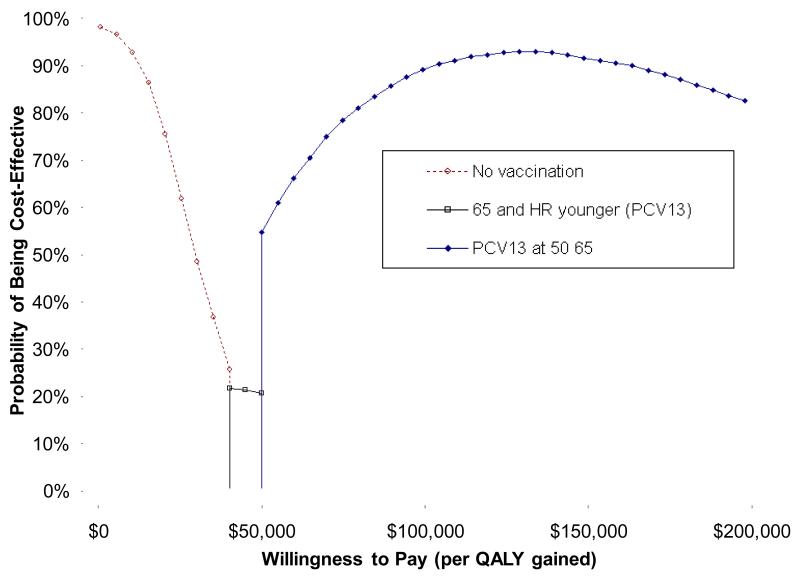
Figure 4. Cost-effectiveness acceptability frontier.
The cost-effectiveness acceptability frontier depicts the strategy with the highest expected net benefit for a given willingness to pay threshold. The no vaccination strategy has the highest expected net benefit at values of ≤$35,000/QALY, PCV13 substituted for PPSV23 in current recommendations is favored from $40,000-50,000/QALY, and PCV13 at ages 50 and 65 is favored at higher willingness to pay thresholds >$50,000/QALY.
Acknowledgments
We thank the Working Group on Pneumococcal Vaccines of the Advisory Committee on Immunization Practices for providing their expert opinion of pneumococcal vaccine efficacy estimates. We also thank Matthew R Moore, MD, MPH and Ruth Link-Gelles for data from the Active Bacterial Core Surveillance System at CDC.
This work was supported by the NIAID (R01 AI076256)
This study was supported by the National Institute of Allergy and Infectious Diseases, Grant No. R01AI076256
Footnotes
Conflicts:
Richard Zimmerman and Mary Patricia Nowalk have a research grant from Merck, Inc., on HPV vaccine
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morb Mortal Wkly Rep. 2010;59(34):1102–6. [PubMed] [Google Scholar]
- 2.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47(10):1328–38. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine? Chest. 2010;138(3):486–90. doi: 10.1378/chest.10-0738. [DOI] [PubMed] [Google Scholar]
- 4.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. Cmaj. 2009;180(1):48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008;(1):CD000422. doi: 10.1002/14651858.CD000422.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369(9568):1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 7.Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2(1) doi: 10.1128/mBio.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 9.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258–61. [PubMed] [Google Scholar]
- 11.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-11):1–18. [PubMed] [Google Scholar]
- 12.Fry AM, Zell ER, Schuchat A, Butler JC, Whitney CG. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine. 2002;21(3-4):303–11. doi: 10.1016/s0264-410x(02)00451-6. [DOI] [PubMed] [Google Scholar]
- 13.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hak E, Grobbee DE, Sanders EA, Verheij TJ, Bolkenbaas M, Huijts SM, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66(9):378–83. [PubMed] [Google Scholar]
- 15.Advisory Committee on Immunization Practices 2011 Feb 23-24; Agenda. http://www.cdc.gov/vaccines/recs/acip/downloads/agenda-feb11.pdf.
- 16.Self-reported pneumococcal vaccination coverage trends 1989 - 2008 among adults by age group, risk group, race/ethnicity, health-care worker status, and pregnancy status. National Health Interview Survey (NHIS); United States: http://www.cdc.gov/flu/professionals/vaccination/pdf/NHIS89_08ppvvaxtrendtab.pdf. [Google Scholar]
- 17.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 18.Zimmerman RK, Lauderdale DS, Tan SM, Wagener DK. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine. 28(39):6470–7. doi: 10.1016/j.vaccine.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg RJ, Larson M, Levy D. Factors associated with survival to 75 years of age in middle-aged men and women. The Framingham Study. Arch Intern Med. 1996;156(5):505–9. [PubMed] [Google Scholar]
- 20.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159(11):1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Vokonas PS. Demographics of the prevalence, incidence, and management of coronary heart disease in the elderly and in women. Ann Epidemiol. 1992;2(1-2):5–14. doi: 10.1016/1047-2797(92)90031-k. [DOI] [PubMed] [Google Scholar]
- 23.SEER age-specific cancer incidence. all sites. http://seer.cancer.gov/faststats/sites.php.
- 24.Arias E. National vital statistics reports. 21. Vol. 58. National Center for Health Statistics; Hyattsville, Maryland: 2010. United States life tables, 2006. [PubMed] [Google Scholar]
- 25.Carbonara S, Monno L, Longo B, Angarano G. Community-acquired pneumonia. Curr Opin Pulm Med. 2009;15(3):261–73. doi: 10.1097/MCP.0b013e3283287c3f. [DOI] [PubMed] [Google Scholar]
- 26.American Lung Association Trends in pneumonia and influenza morbidity and mortality. 2010 http://www.lungusa.org/finding-cures/our-research/trend-reports/pi-trend-report.pdf.
- 27.Rubin JL, McGarry LJ, Strutton DR, Klugman KP, Pelton SI, Gilmore KE, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–43. doi: 10.1016/j.vaccine.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 28.Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28(31):4955–60. doi: 10.1016/j.vaccine.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko FS, McEllistrem MC, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: A cost-effectiveness analysis. Vaccine. 2008;26(11):1420–31. doi: 10.1016/j.vaccine.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Wyrwich KW, Fihn SD, Tierney WM, Kroenke K, Babu AN, Wolinsky FD. Clinically important changes in health-related quality of life for patients with chronic obstructive pulmonary disease. Journal of General Internal Medicine. 2003;18(3):196–202. doi: 10.1046/j.1525-1497.2003.20203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine MJ, Stone RA, Singer DE, Coley CM, Marrie TJ, Lave JR, et al. Processes and outcomes of care for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study. Arch Intern Med. 1999;159(9):970–80. doi: 10.1001/archinte.159.9.970. [DOI] [PubMed] [Google Scholar]
- 32.Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA. 1999;281(3):243–8. doi: 10.1001/jama.281.3.243. [DOI] [PubMed] [Google Scholar]
- 33.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical Care. 1998;36(6):778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Annals of Internal Medicine. 2003;138(12):960–8. doi: 10.7326/0003-4819-138-12-200306170-00007. [DOI] [PubMed] [Google Scholar]
- 35.CDC Vaccine Price List. http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm.
- 36. [Accessed Nov 16, 2010];Medicare Physician Fee Schedule Look-up (CPT 90471) 2006 http://www.cms.hhs.gov/apps/pfslookup/Datayear.
- 37.Healthcare Cost & Utilization Project (HCUP) http://www.ahrq.gov/data/hcup/
- 38.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5(2):83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 39.Lu PJ, Nuorti JP. Pneumococcal polysaccharide vaccination among adults aged 65 years and older, U.S., 1989-2008. Am J Prev Med. 2010;39(4):287–95. doi: 10.1016/j.amepre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Census Bureau National Population Estimates for the 2000s. http://www.census.gov/popest/national/asrh/files/NC-EST2009-ALLDATA-R-File14.csv.
- 41.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14(3):259–65. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
- 42.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Cmaj. 1992;146(4):473–81. [PMC free article] [PubMed] [Google Scholar]
- 43.Braithwaite RS, Meltzer DO, King JT, Jr., Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 44.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 45.Joint Committee on Vaccination and Immunisation JCVI statement on the routine pneumococcal vaccination programme for adults aged 65 years and older. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_125122.pdf. [Google Scholar]
- 46.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 47.Hansen J, Black S, Shinefield H, Cherian T, Benson J, Fireman B, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25(9):779–81. doi: 10.1097/01.inf.0000232706.35674.2f. [DOI] [PubMed] [Google Scholar]
- 48.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 49.Millar EV, Watt JP, Bronsdon MA, Dallas J, Reid R, Santosham M, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47(8):989–96. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 50.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 51.Lu PJ, Nuorti JP. Uptake of pneumococcal polysaccharide vaccination among working-age adults with underlying medical conditions, United States. Am J Epidemiol. 2009 doi: 10.1093/aje/kwr376. in press. [DOI] [PubMed] [Google Scholar]