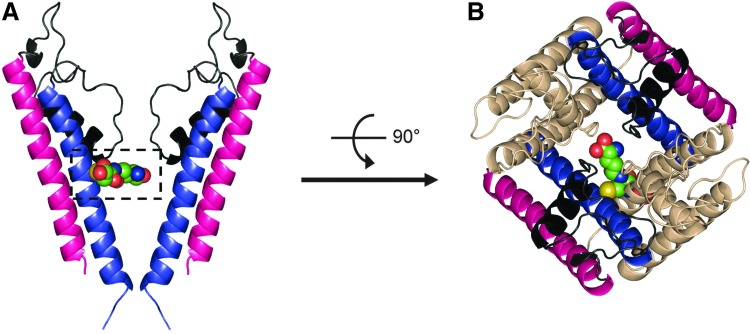
FIG. 4.
Structural insights of Kir4.1-Kir5.1 channel S-glutathionylation. (A) Side view of structural model of Kir4.1–5.1 (pore-forming domain) in the closed state (two opposing Kir5.1 monomers are shown). Outer helix of Kir5.1 is colored magenta and inner helix of Kir5.1 is colored blue. A glutathionyl moiety is bound to one Cys158 residue of Kir5.1 via disulfide bond. (B) Extracellular view of glutathionyl moiety adducted to the Cys158 residue of the inner helix in Kir4.1-Kir5.1 channel. The two Kir4.1 subunits are colored wheat. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars