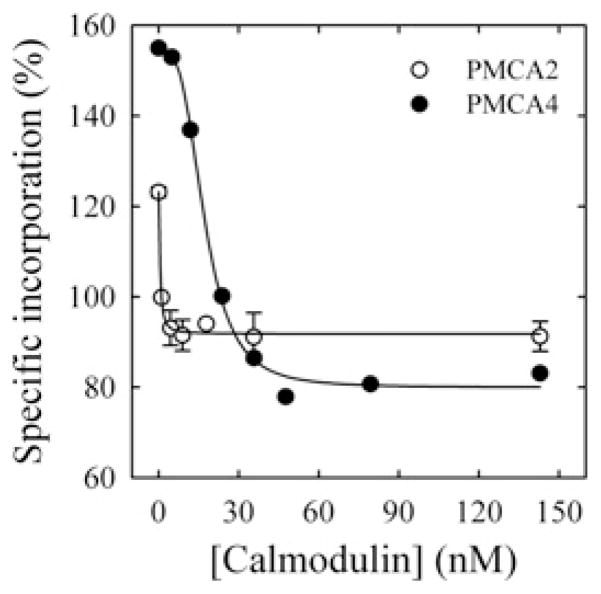
Figure 3. CaM-dependence of the incorporation of [125I]TID-PC/16 to PMCA4 (●) and PMCA2 (○).
Incorporation of [125I]TID-PC/16 was determined in a medium containing 100 μM Ca2+ at 37°C. Eqn (2) was fitted to the experimental points, where [PCB] is the percentage relative to EGTA condition of [125I]TID-PC/16 bound to PMCA at a given concentration of CaM; [PCmin] is the minimal amount (%) of [125I]TID-PC/16 bound to PMCA (at saturating concentration of CaM), [PC0] is the maximal amount (%) of PC bound to PMCA (at zero [CaM]) and K d(CaM) is the concentration of CaM for half-maximal binding to PMCA. This resulted in the following values: [PCmin] = 79.9 ± 1.9 %, [PC0] = 154.5 ± 2.2 %, n = 3.3 ± 0.4 and K d(CaM) = 17.6 ± 0.9 nM for PMCA4; and [PCmin] = 91.8 ± 0.9 %, [PC0] = 123.2 ± 1.4 %, n = 1.4 ± 0.6 and K d(CaM) = 0.52 ± 0.08 nM for PMCA2.