Abstract
The use of systemic chemotherapeutic drugs and molecular-targeted therapies in the treatment of patients with locally advanced or metastatic lung cancer has its limitations due to the associated acute and cumulative dose limiting toxicities and acquisition of drug resistance. Prevention and therapeutic intervention by dietary agents including nutraceuticals which are non-toxic, cost-effective, and physiologically bioavailable, are emerging approaches in lung cancer management. In this regard, silibinin, a natural flavonolignan, has been rigorously evaluated for the prevention and growth control of lung cancer through extensive in vitro and in vivo studies. Successful studies conducted so far, have established that silibinin is effective both alone and in combination with other agents (e.g. chemotherapeutic and epigenetic agents) in significantly inhibiting the growth of lung cancer cells. In vivo, its effects have been shown to be mediated through inhibition of proliferation, angiogenesis and epigenetic-related events. Therefore, the present review focuses on encompassing the efficacy and mechanisms of silibinin against lung cancer.
Keywords: Lung cancer, Silibinin, Phytochemicals, Chemoprevention
1. Lung Cancer
Lung cancer is the mostly deadly malignancy, and also the most common cause of death from cancer. Statistical estimates by American Cancer Society for 2012 indicate 226,160 new lung cancer cases and 160,340 associated deaths in United States (US) alone, suggesting that more individuals are likely to die of lung cancer, than of colon, breast and prostate cancers combined [1]. Poor prognosis is due to the late diagnosis of lung cancer at an advanced stage; patient mortality being highly associated with metastatic spread of the cancer within the lung and to distant sites [2]. Majority of lung cancers are epithelial in origin, arising from proximal respiratory tract epithelium including the bronchi. Lung cancer is clinically divided into two distinct classes, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) [3]. This pathological classification helps distinguish these classes on the basis of their tendency to metastasize and response to existing therapies, in order to predetermine their clinical management [4]. Importantly, ~75% of lung cancers are NSCLC with strong causal association with smoking. Though NSCLC is less metastatic than SCLC, it is less responsive to chemotherapy [4] and hence has been the main focus of current research on investigating new drugs for its therapy.
2. Management and Limitations of Existing NSCLC Therapies
NSCLC is classified into three primary stages, namely local (IA, IB, IIA), locally advanced (IIB, IIIA, IIIB) and the advanced (IIIB, IV) stages. These stages are further stratified depending on tumor size, lymph node status, and metastases of the tumor [5,6]. Surgery is the most successful option for cure for NSCLC patients diagnosed with early stages (I–IIIA). The 5-year survival rate of patients, post-surgery, varies from <5% (Stage III–IV), 23% (Stage IIIA) to 67% (Stage IA) [5–7]. Patients with resected lung cancer have a high-risk of relapse, and the results of the four randomized trials including International Adjuvant Lung Cancer Trial (IALT), the National Cancer Institute of Canada intergroup study, the Cancer and Leukemia group B (CALGB) trial, the Adjuvant Navelbine International Trialist Association Trial have supported the use of adjuvant chemotherapy after complete resection of early stages (IIA–IIIA) [8]. Chemotherapy, in parallel with radiotherapy, has also shown modest survival benefits in patients with inoperable locally advanced and disseminated form of this disease [9,10]. Before 1990, only a few drugs including alkylating agents (e.g. cisplatin, ifosfamide), DNA crosslinking agents (e.g. mitomycin C), mitotic inhibitors (e.g. vinblastine, vindesine), and topoisomerase inhibitors (e.g. etoposide) were used against NSCLC; of which, the most effective chemotherapeutic agents were the platinum-based alkylating agents such as cisplatin [8]. In 1995, patients with advanced disease, treated with platinum based chemotherapy, showed improvement with increased median survival of 1.5 months [10]. Following that, new chemotherapeutic agents such as gemcitabine, vinorelbine, paclitaxel, and docetaxel have been successfully used against NSCLC. To improve the outcome for patients, these agents have been combined with either cisplatin or carboplatin, especially, in the treatment of the cases with advanced stages of NSCLC [9]. Platinum-based chemotherapy is associated with significant toxicity and therefore non-platinum-(e.g. taxane-based) treatment regimens, have also been tested against NSCLC [11]. Moreover, several other targeted therapies are being clinically evaluated for the treatment of lung cancer, namely (i) EGFR inhibitors (e.g. Gefitinib (IressaR), Erlotinib (TarcevaR), (ii) VEGF/VEGFR inhibitors e.g. Bevacizumab (AvastinR), Vandetanib (ZactimaR), (iii) PI3K/Akt/mTOR inhibitors e.g. Rapamycin (RapamuneR), (iv) proteasome inhibitors e.g. Bortezomib (VelcadeR), (v) HDAC inhibitors e.g. Vorinostat (ZolinzaR) [12]. Conclusively, the use of systemic chemotherapeutic drugs and molecular targeted therapies in the treatment of patients with locally advanced or metastatic NSCLC is limited due to the associated acute and cumulative dose limiting toxicities and acquisition of drug resistance [13,14]. This fact further stresses the importance of developing additional effective strategies [15,16]. Prevention and therapeutic intervention by phytochemicals which are non-toxic, cost-effective, and physiologically bioavailable are emerging approaches in cancer management. Several advantages associated with these, over the conventional treatment modalities like chemotherapy and radiation therapy, qualify these agents as ideal candidates in chemoprevention strategies [17].
3. Cancer Chemoprevention
The concept of multi-step carcinogenesis implies that cancer is a complex disease that involves a series of events involving genetic and epigenetic changes at the molecular level that aid in the transformation of normal epithelial cells through stages like hyperplasia, dysplasia, carcinoma in situ to invasive carcinoma [18–20]. The primary aim of chemoprevention studies is intervention within this multi-step process of carcinogenesis [18]. The genesis of chemoprevention studies dates back to 1976, when Michael Sporn, coined the term chemoprevention, defining it as the use of natural and synthetic agents to inhibit, delay or reverse the process of carcinogenesis [21]. A suitable chemopreventive agent should satisfy measures for: (i) Primary prevention- in high risk populations (e.g. individuals with a smoking history), (ii) Secondary prevention-in patients with precursor lesions (e.g. atypical adenomatous hyperplasia), and (iii) Tertiary prevention-in cancer patients, post therapy (e.g. development of secondary cancer in patients treated primarily for lung cancer) [22,23].
4. Lung Cancer Chemoprevention
The major focus of lung cancer prevention studies is to reduce its incidence, by encouraging healthy practices such as smoking cessation amongst current smokers [24]. But statistically, the number of lung cancer-related deaths continues to rise, with 50% of cases known to occur in former smokers [18]. Alternatively, chemoprevention is a plausible secondary prevention approach targeting both high-risk former and current smokers by the use of agents that can interfere with the initiation, promotion and progression of cancer [24,25]. Initially, diet-related epidemiologic case control and cohort studies helped in identifying potential chemopreventive agents against the development of lung cancer [26]. Through 1980–1990, several nutrients including β-carotene, vitamin A (retinol, retinyl palmitate, isotretinoin) and vitamin E were extensively investigated. In this regard, phase III primary chemoprevention based trials including the α-tocopherol, β-carotene (ATBC) trial, the β-carotene and Retinol Efficacy Trial (CARET), and the Physician’s Health study were conducted [23]. In the Finland based ATBC trial (1985–1993), male current smokers (smoking ≥5 cigarettes per day) aged 59–60 years were administered either a placebo or a daily supplementation of 50 mg of α-tocopherol (form of vitamin E) or 20 mg of β-carotene (a precursor of vitamin A) or both. The study concluded with disappointing results, with both agents being unable to reduce the risk of lung cancer [27,28]. The U.S. based CARET trial, included smokers and former smokers [aged 50–69 years, with a history of at least 20-pack years; i.e.1 pack year = number of packs cigarettes per day × number of years smoked] as well as male asbestos-exposed workers. Participants were supplemented with β-carotene (30 mg) plus retinol (25,000 IU) versus placebo for every day, for 4 years [26,28].Unfortunately, similar to the ATBC trial results, subjects receiving β-carotene had 28% increase in lung cancer incidence compared to the controls. Former smokers receiving β-carotene supplementation were 20% less likely to develop lung cancer, suggesting that β-carotene can prevent the development of lung cancer, but the effect would eventually be overcome due to continued cigarette smoke exposure. Moreover, 8% and 17% increase in mortalities, associated mainly with heavy cigarette smoking, were observed, respectively, in the ATBC and CARET trials [18]. In the Physicians Health Study, the effect of β-carotene (50 mg every other day for 12 years) on lung cancer incidence was determined in healthy male non-smoking U.S. physicians. [20]. The results from this study indicated no significant protective effects. Four phase IIb, secondary chemoprevention based trials were attempted in humans by evaluating the efficacy of α-tocopherol, β-carotene, retinol and isotretinoin in smokers [19]. Agents such as isotretinoin (13-cis-retinoic acid) and fenretinide (N-(4-hydroxyphenyl) retinamide) were tested and shown to be inefficacious in reversing bronchial squamous metaplasia/dysplasia [29]. Another trial tested the synthetic retinoid etretinate for the reversal of sputum atypia in smokers, which yielded negative results. In the same study, a significant decrease in metaplasia was noted to correlate with smoking cessation, in both the treatment and the placebo groups [19]. Despite few failures, some interesting trials merit further exploration. For example, a preliminary intervention trial showed that folate and vitamin B12 intake could improve bronchial squamous metaplasia in smokers [19]. Also, the results of a phase IIb trial reported that anethole dithiolethione can benefit in the treatment of bronchial dysplastic lesions in smokers [19]. Recently, in a phase I study, myo-inositol effectively regressed bronchial dysplasia in the treatment group compared to the controls [30]. With respect to tertiary chemoprevention trials of lung cancer, three major phase III studies were performed. The first one was an adjuvant trial of retinyl palmitate (vitamin A) (300,000 IU/day for 12 months) to prevent the formation of second primary tumors (SPT) in Stage I NSCLC patients, which produced positive results [18]. Overall, the results from this trial indicated the advantage of the treatment of high-dose vitamin A in effectively reducing the number of new tobacco-related primary tumors as well as to improve the condition in patients curatively resected for early stage lung cancer [18]. The EUROSCAN (European Study on Chemoprevention of Vitamin A and N-acetylcysteine) trial concluded by showing no improvement in survival nor incidence of SPTs in patients with NSCLC with a smoking history [18,28,29]. An identical result was observed in the NCI intergroup phase III trial, where low dose isotretinoin failed to improve the rates of SPTs or mortality, after definitive resection in stage I NSCLC patients. Although isotretinoin was harmful in current smokers, the outcome was shown to be beneficial in cases of never smokers [31].
In brief, previously conducted lung cancer chemoprevention trials have not shown any benefit in reducing the risk of lung cancer. Most of these trials were based on the use of pleiotropic natural agents in populations, predominantly characterized by their smoking status [26]. Though these trials have been unsuccessful, they have helped in identifying and outlining strategies that can be further implemented to effectively control lung cancer. Such criteria include: (i) identification of molecular targets that can be modulated to achieve risk reduction, (ii) use of targeted agents, alone and/or in combination, (iii) identification of high-risk populations, (iv) development of reliable biomarkers, and (v) validation of secondary endpoint biomarkers (e.g. combined assessment of histology from lung biopsy specimens and targeted biomarkers) [28,32]. At present, no definite biomarkers for lung cancer prevention trials have been established. Certain promising markers such as Ki67, p53, EGFR expression, and gene methylation are being evaluated and require further validation [23,33]. Currently, a significant number of chemoprevention agents are being evaluated, mostly in ongoing or completed phase II trials that have been initiated on the basis of epidemiological and preclinical data. These phase II intermediate endpoint trials are being conducted to screen and identify efficacious agents that can be further tested in large and expensive phase III trials [18,22,33]. Chemoprevention of lung cancer still remains a promising strategy with the advent of several natural and synthetic compounds including promising dietary agents that need to be further investigated. In this regard, several phytochemicals, for example: curcumin, genistein, grape seed extract, resveratrol, and silibinin are undergoing critical pre-clinical experimental investigations for their efficacy against lung cancer. It is however, difficult as well as pre-mature to predict which phytochemical amongst these will be more efficacious in the clinical trials. Nevertheless, silibinin, because of its proven clinical use and pleotropic mechanism of action, does indeed, stand a fair chance of being the first choice amongst these nutraceuticals for use against lung cancer.
5. Silibinin in Chemoprevention of Lung Cancer
Silibinin (C25H22O10, molecular weight (wt.), 482.44), a flavonolignan, is the major biologically active constituent of silymarin, isolated from the dried fruits and seeds of Silybum marianum (milk thistle) as shown in Fig. 1. It has shown significant efficacy in inhibiting or delaying both tumor initiation- and promotion-related events in various pre-clinical cancer models including that of skin, prostate, colorectal, and lung cancer [34–37]. Silibinin has a long history of human use, and acute and chronic doses of silibinin administration in animals and humans have shown no significant toxicity. Moreover, no LD50 for silibinin has been reported in rodent studies [34–37]. In prevention/intervention settings, silibinin intake has been proven to be advantageous due to its wide usage as a popular dietary supplement, well tolerability and minimal toxicity [34–37]. Milk thistle extract (MTE) has been used since centuries for hepatic disorders and used clinically against toxic mushroom poisoning [34,38–41]. Currently, MTE or silibinin is not approved for medicinal use in the USA, but it is commonly used as a nutraceutical/dietary supplement in Europe, Asia and USA. Legalon, sold by Rottapharm/Madaus (contains standardized 80% silymarin) is widely used in the USA, and also has been used in clinical trial studies [42]. Standardized MTE consists of 70–80% of silymarin, a complex mixture of 7 flavonolignans and 1 flavonoid. Silymarin was first isolated from the seeds of milk thistle in 1968 and its constituents include silibinin (50–60%), silichristin (20%), silidianin (10%), isosilibinin (5%), taxifolin and quercetin [42].The remaining 20–30% of MTE comprises of polyphenols and aliphatic fatty acids. Silibinin, the major constituent of silymarin, is further composed of 1:1 mixture of two diastereoisomers- silybin A and silybin B.
Fig. 1.
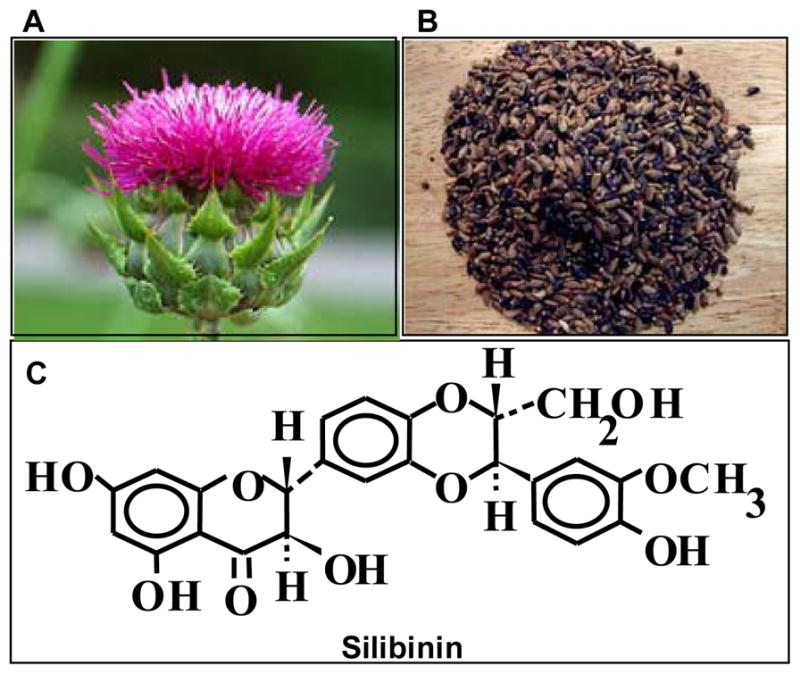
Milk Thistle. (A) Silybum marianum (Milk Thistle) plant, Family: Asteraceae. (B) Seeds of Milk thistle plant. (C) Chemical structure of silibinin - the principle bioactive constituent of silymarin (milk thistle extract) isolated from the dried fruits and seeds of milk thistle.
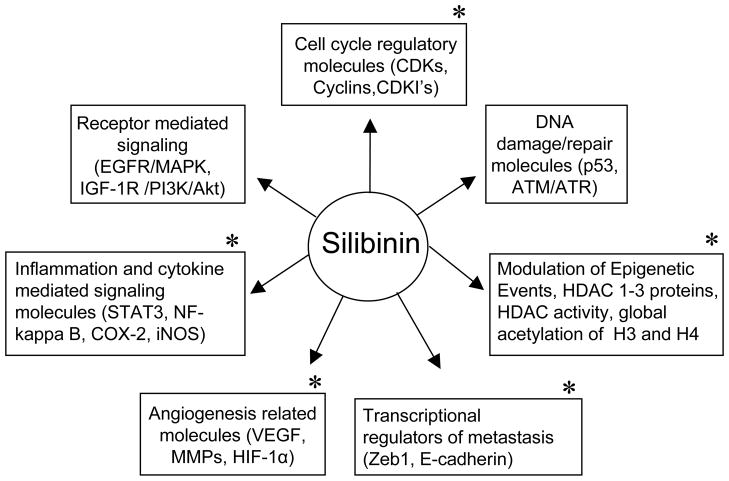
Amongst the various cancer models, the effects of silibinin have been also rigorously assessed in the prevention and growth control of lung cancer through extensive in vitro and in vivo studies conducted especially in our laboratory [43–47]. Therefore, the present review focuses on encompassing the efficacy and mechanisms of silibinin against lung cancer (Fig. 2).
Fig. 2.
Major molecular targets of silibinin involved in its efficacy against epithelial cancers. *, Key molecules that are targeted by silibinin in its chemopreventive and anti-cancer efficacy against lung cancer.
A) Efficacy of Silibinin in Lung Cancer Cell Lines
Initial studies have shown that in lung cancer, silibinin treatments (25–100 μM) demonstrate significant efficacy in SHP-77 (SCLC) and A549 (NSCLC) cells [48]. Apart from inducing cell growth inhibition, silibinin also caused alterations in cell cycle checkpoints and a dose-dependent increase in apoptotic death in these cell lines. Recently, the effects of silibinin in different subtypes of NSCLC (H1299, H460 and H322) cells have also been characterized [44]. Follow-up studies (as detailed in the next section), revealed that silibinin is efficacious in inhibiting primary lung tumor growth and progression in mice, accompanied by down-regulation of inducible nitric oxide (iNOS) expression in treated tumors. iNOS is one of the three major isoforms of nitric oxide (NO), a free radical that is known to be associated with the growth and progression of lung tumors. iNOS expression/activity in lung tumors is indicative of the angiogenic and metastatic potential of tumors [45].
Chittezhath et al., investigated the role of silibinin on the cytokine (IFN-γ + IL-1β + TNF-α) mixture-activated signaling cascades, which are known to promote iNOS expression, employing A549 cells [43]. Altogether, silibinin treatments inhibited iNOS expression in these cells, it was consequentially due to the down-modulation of the multiple upstream cytokine-induced signaling pathways that were targeted in response to silibinin treatment [43]. Recently, the effects of silibinin in tumor-derived mouse lung epithelial LM2 cells were also reported [47]. Pre-treatment with silibinin consistently decreased cytokine mixture (TNF-α + IFN-γ) induced activation of STAT3 (Tyr-705 and Ser-727), STAT1 (Tyr-701), ERK1/2 as well as NF-κB-DNA binding. Cytokine mixture also strongly induced cyclooxygenase-2 (COX-2) and iNOS expression, which was significantly inhibited with silibinin treatments. Altogether these studies identified silibinin’ efficacy in modulating key signaling cascades involved in the regulation of its major targets such as iNOS and COX-2 [43,47]. In metastasis related events, silibinin has been shown to target epithelial to mesenchymal transition (EMT) by up regulating epithelial markers, while concomitantly inhibiting expression of mesenchymal markers (e.g. snail, fibronectin, vimentin etc.) [49,50]. Chen et al., reported the protective effect of oral silibinin (200 mg/day/kg of body wt.) against the metastasis of Lewis lung carcinoma cells subcutaneously injected in C57BL/6 male mice [51]. In another study, Chu et al., showed the down-modulation of proteolytic enzymes like MMPs and u-PA expression in silibinin-treated A549 cells [52]. These results are significant since lung cancer is a highly aggressive disease, in which cells metastasize very early within the lungs and to distant sites in the body.
B) Efficacy of Silibinin in Combination with Other Agents against Lung Cancer
Based on the promising efficacy of silibinin as a single agent in lung cancer, Singh et al., conducted a study using a combination of silibinin and doxorubicin in A549 cells. In this study, silibinin (60 μM) in combination with doxorubicin (25 nm) was synergistically effective in terms of causing prominent cell growth inhibition (85%), as compared to the efficacy of either agent alone [53]. The combination synergistically increased apoptotic cell death and inhibited NF-κB-based chemoresistance to doxorubicin [53]. Overall, these in vitro findings suggested that silibinin is efficacious in increasing the therapeutic efficacy of doxorubicin [53]. Further, this promising combination was also tested in A549 tumor xenografts in athymic nude mice, which will be detailed in the next section. Rho et al., has reported in a recent study, that the addition of silibinin to EGFR-TKIs is promising in overcoming the Threonine (Thr) 790 mutation (T790M)-mediated drug resistance to EGFR-TKIs [54]. In this study, silibinin treatments (100 μM) in a time-dependent manner, inhibited the activity of EGFR family (EGFR, ErbB2, and ErbB3) in EGFR mutant cells, but not in the cell lines harboring wild-type EFGR. Additional mechanism-based studies showed that silibinin inhibits homo- and hetero-dimerization of EGFR mutant cells in a ligand-dependent and -independent manner. Furthermore, in T790M-containing resistant cells, silibinin treatments increased the efficacy of gefitinib and erlotinib to down-regulate EGFR activity and its downstream signaling pathways (Akt and ERK) as well as to suppress cell growth [54]. Further, a combination of indole-3-carbinol (a glucobrassicin derivative present in cruciferous vegetables) and silibinin has also been tested against lung cancer, which will be detailed in the next section.
C) Efficacy of Silibinin in Preclinical Models of Lung Cancer
(i) Efficacy of Silibinin in Lung Cancer Xenograft Model
Oral feeding of silibinin (200 mg/kg body wt., 5 days/week, for 33 days) along with doxorubicin (4 mg/kg body wt., 1 day/week, a total of 4 doses) showed synergistic growth suppression of A549 xenograft in athymic nude mice [53]. The combination inhibited cell proliferation, angiogenesis and enhanced apoptosis in tumors. In this study, silibinin not only enhanced the therapeutic response of doxorubicin, but also strongly reduced doxorubicin-induced systemic toxicity, when the drugs were co-administered in mice [53]. In another study by Rho et. al., daily oral co-administration of silibinin (200 mg/kg) and erlotinib (100 mg/kg) for 24 days resulted in irreversible suppression of tumor growth and induction of apoptosis in erlotinib resistant PC-9 tumors in SCID mice [54]. These effects were accompanied by changes at the molecular levels where the drug combination effectively reduced EGFR and Akt activity in tumors. A significant decrease in 18F-fludeoxyglucose uptake was also observed in tumors exposed to co-treatments. The findings of this study implicated the use of silibinin in combination with EGFR-TKI for preventing the growth of cells, with pre existing T790M mutation or for the treatment of patients with T790M-mediated acquired resistance to EGFR TKIs [54].
(ii) Efficacy of Silibinin in Carcinogen-Induced Mouse Models of Lung Cancer
The in vivo efficacy of silibinin on tumor growth has been rigorously investigated in mouse models of lung cancer. The chemopreventive potential of dietary silibinin on urethane-induced lung tumors was reported for the first time by Singh et al. In this study, 6-week old A/J mice were challenged with a single intraperitoneal (i.p) injection of urethane (1 mg/g body wt.) to induce pulmonary tumors [46]. Two weeks after the urethane injection, mice were randomly divided and assigned to either the control diet (AIN76A diet) or diet containing varying doses of silibinin (0–1% wt/wt) for an additional 18–27 weeks, at which time they were accordingly sacrificed. The results of this study revealed that urethane-injected mice that were exposed to dietary silibinin had statistically significantly lower lung tumor multiplicities than the urethane-injected control mice. The clinically relevant observation was that mice fed-with 1% wt/wt silibinin in diet for 18 weeks had 93% fewer large (i.e. 1.5–2.5-mm-diameter) lung tumors than the control mice, suggesting that silibinin prevented the urethane-induced lung tumors from growing beyond a small size, i.e. from avascular to vascular stages, as assayed by tumor size [46]. Further, lung tumors of silibinin-fed mice, showed decreased proliferative index than lung tumors of control mice. Moreover, immunostaining for angiogenesis related markers showed that tumor microvessel density (platelet endothelial cell adhesion molecule-1 (PECAM-1/CD-31) and lung tumor expression of VEGF were significantly reduced with silibinin treatments. Silibinin treatments also caused a significant decrease in iNOS and COX-2 expression, two enzymes that promote angiogenesis by inducing VEGF expression [46]. The results from this study are highly significant, given the fact that majority of current and former smokers have small (< 2 mm) nodules, advocating the development of chemoprevention strategies that aim to target angiogenesis-based mechanisms to control the growth of lung cancer.
Following this study, Tyagi et al., assessed the chemotherapeutic efficacy of oral silibinin on urethane-induced advanced lung tumors in the same strain of mice [55]. After 32 weeks post-urethane injection (1 mg/g body wt., ip), mice were randomly divided into 2 groups and administered either saline (control) or silibinin (742 mg/kg body wt., 5 days/week) by oral gavage for 10 weeks. The oral dose of silibinin chosen for this study was equivalent to the 1% wt/wt dietary silibinin as extrapolated from the study conducted by Singh et al. [46]. Mice were sacrificed at the completion of 43 weeks post-urethane injection, where silibinin-treated group showed 33% fewer tumors, significant decrease in lung adenocarcinoma multiplicity, tumor burden, tumor size, and a consistent decrease in the number of larger tumors compared to the controls [55]. Though proliferation and apoptosis were only marginally affected; marked suppression of tumor angiogenesis was observed in silibinin-treated set of tumors. While silibinin did not affect tumor microvessel density, it significantly reduced microvessel size and the formation of new microvessels in the tumors [55]. Furthermore, silibinin-treated tumors showed a strong decrease in the infiltrated tumor associated macrophages (TAMs) in the lung as well as reduced levels of pro-inflammatory and pro-angiogenic cytokines (e.g. IL-13, TNF-α), concomitant with increased levels of angiogenic inhibitors such as angiopoietin-2 (Ang-2) and ang-receptor tyrosine kinase (Tie-2) that are known to regulate vessel stabilization and angiogenesis. Apart from inhibiting the activation of the transcription factors (HIF-1α, NF-κB and STAT3), silibinin treatments also increased the levels of enzymes inhibiting metalloproteinases such as TIMP-1 and TIMP-2 [55]. Despite significant changes in angiogenesis-related markers, silibinin treatments did not alter the expression of VEGF, iNOS and COX-2, although significant changes in the levels of these proteins was observed in the silibinin-treated tumors in the previous study [46]. This suggested that different mediators of angiogenesis are involved at different stages of tumor growth and progression, and that silibinin modulates their expression differentially at different stages of lung carcinogenesis to inhibit urethane-induced lung tumors.
Another study assessed the efficacy of silibinin, alone and in combination with indole-3-carbinol against NNK-induced lung adenocarcinoma in A/J mice [56]. NNK-exposed mice were treated with silibinin (7 μmol/g), indole-3-carbinol (10 μmol/g) or both. The combinatorial treatment was efficacious in reducing tumor multiplicity and growth of NNK-induced lung tumors. In vivo molecular analysis showed that the combination treatment synergistically modulated the levels of phospho-Akt, phospho-ERK and cyclin D1 as well as induced a significant increase in poly (ADP-ribose) polymerase (PARP) cleavage as compared to single agents alone. Parallel to these findings, the potential effects of silibinin was assessed against benzo (a) pyrene-induced lung tumorigenesis in A/J mice [57]; however, dietary feeding of silibinin, at doses of 0.05% and 0.1% wt/wt did not show significant efficacy in inhibiting pulmonary adenoma formation and growth [57], suggesting that the efficacy of silibinin in inhibiting lung tumorigenesis differs in varying carcinogen-induced mouse models of lung cancer.
(iii) Efficacy of Silibinin in Urethane-Induced Lung Tumorigenesis in B6/129 Wild-type and iNOS−/− Mice
As detailed earlier in the text, studies have shown that silibinin modulates iNOS levels in its angiopreventive efficacy against urethane-induced lung tumorigenesis. To understand the importance of iNOS inhibition in silibinin-mediated chemopreventive effects, Ramasamy et. al., investigated the efficacy of silibinin on urethane-induced lung tumorigenesis in B6/129 wild-type (WT) mice and iNOS −/− mice. Male B6/129-Nos2tm1Lau (iNOS−/−) and B6/129PF2 WT mice (5–6 weeks old) were injected with 1 mg/g body wt. urethane (i.p.) once weekly for 7 consecutive weeks [45]. Evaluation of lung tissue and tumors showed prominent adenomas in B6/129 mice after the completion of 7 weekly urethane injections. Next, the mice were divided into two groups and orally gavaged with either 0.2 ml vehicle (0.5% (w/v) carboxymethyl cellulose and 0.025% Tween 20 in distilled water) or silibinin (742 mg/kg body wt.) in vehicle, for 5 days/week for total of 18 weeks, and at the end of treatment regimen, mice were sacrificed. Analysis of lung tumors showed that genetic ablation of iNOS decreased urethane-induced tumor multiplicity by 87%, as compared to WT mice. The lung tumor size in WT mice varied from 0.5–4.5 mm, whereas in iNOS −/− mice, lung tumors were <1 mm in diameter (with an overall 82% reduction in size). Furthermore, in iNOS −/− mice, no significant difference in number and size of urethane-induced tumors was observed between the control and silibinin-treated groups; however, silibinin inhibited urethane-induced lung tumorigenesis in WT mice, showing reduced lung tumor multiplicity by 71%. With respect to the size of the lesions, a significant reduction of 67% (< 1 mm lesions), 62% (1.0–1.5 mm lesions) and almost complete inhibition of lesions ranging in diameter between 1.5–2.5 mm and >2.5 mm was recorded [45]. The number and sizes of lung tumors was affirmed by subjecting lung tumors of WT mice (from both control and silibinin treatments) to real-time micro-CT scanning, which showed similar results, consistent with the direct ex vivo measurements of tumors, conduced at the termination of the experiment. Unlike the case in iNOS−/− mice, immunostaining of lung tumors of control and silibinin-treated tumor-bearing WT mice showed that silibinin treatments strongly reduced iNOS levels by 57% [45]. This result was in concert with the previous findings, further validating that silibinin targets iNOS, while mediating its anti-tumor efficacy against urethane-induced lung tumorigenesis [46]. In silibinin-treated groups, the decrease in number and sizes of lung tumors was shown to be through the moderate reduction of tumor cell proliferation and neoangiogenesis as confirmed by immunostaining of tumor samples for PCNA and nestin. A robust decrease in the levels of pro-angiogenic molecules such as VEGFR-2 (a major receptor for VEGF) and reduced expression and nuclear localization of transcription factors like p-STAT3 (Ser-727), and p65 NF-κB (Ser-276) was observed in silibinin-treated groups. Therefore, the findings of this study enumerated that silibinin primarily exerts its chemopreventive and angiopreventive effects through the modulation of iNOS expression in urethane-induced lung tumors [45].
D) Efficacy of Silibinin Alone and in Combination with Epigenetic Therapies in NSCLC
Targeting the enzymes that catalyze DNA methylation [e.g. DNA methyltransferases (DNMTs)] and covalent post-translational histone modifications [e.g. histone deacetylases (HDACs)] have been recognized, fairly recently, as potential strategies for chemoprevention and treatment of malignancies including NSCLC [58–60]. In this regard, while the utility of silibinin in inhibiting pre-malignant and established stages of lung cancer is ascertained, its possible epigenetic effects were only recently investigated [61]. In this regard, the effects of silibinin on HDAC levels, particularly the subset of HDACs (Class I HDACs) that are known to regulate biological processes [62] such as cell proliferation and death were assessed [61]. Apart from indirectly regulating total enzyme levels, silibinin treatments notably reduced HDAC (1, 2 and 3) protein levels and caused accumulation of acetylated histones (H3 and H4) in total cellular chromatin [61]. Given the limited efficacy of HDAC inhibitors (HDACi) as mono-therapies against solid tumors, their significant toxicities and various mechanisms of resistance, [63,64], it was rationalized that the full therapeutic potential of HDACi to effectively target NSCLC in vitro and in vivo could perhaps be best realized in combination with silibinin. These studies further revealed that combinations of HDCAi [Trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA)] with silibinin synergistically increased the cytotoxic effects of these single agents, which was associated with enhanced p21 promoter-associated histone (H3 and H4) acetylation states that led to a dramatic increase in p21 protein and mRNA levels which limited the supply of cyclin B1, causing a significant G2/M arrest by the combination treatment, followed by apoptotic cell death. These combinations also caused similar epigenetic modulations in vivo, together with a marked reduction in xenograft growth of NSCLC H1299 cells (Agarwal R, unpublished data) Altogether, these studies strongly suggested that silibinin can be developed in combination therapy with HDACi in order to improvise their efficacy and to achieve better clinical responses in NSCLC patients. Further, the potential effect/s of silibinin treatment alone as well as in combination with epigenetic therapies (i.e. either a HDAC or a DNMT inhibitor) on the differential levels of E-cadherin in a range of NSCLC cells was also determined [65]. The significance of this work originated from the fact that E-cadherin is differentially down regulated in NSCLC cells due to epigenetic modulations and its loss of function is thereby recognized as one of the primary factors responsible for EMT and metastasis of lung cancer cells [66]. Silibinin treatment alone as well as in combination with epigenetic drug/s, led to a synergistic increase/restoration of E-cadherin protein expression and a marked reduction in the levels of Zeb1, which is a major transcriptional repressor of E-cadherin in NSCLC cells [65]. In terms of its biological effects, silibinin treatment alone as well as in combination with HDAC or DNMT inhibitors irreversibly reduced the invasion/migratory potential of NSCLC cells, suggesting that the enhanced expression and/or re-expression of E-cadherin by these combinatorial treatments significantly down modulate these EMT-related events [65]. These results were highly significant, given the fact that E-cadherin expression has been used as a clinical biomarker, in predicting responses to conventional therapies like EGFR-TKIs in lung cancer [66,67]. Altogether, these studies have provided a better picture of silibinin’ mechanism of action as a chemopreventive and an anti-cancer agent.
6. Summary
Lung cancer is the leading cause of cancer-related mortalities worldwide, despite the advent of treatment options such as molecular-targeted agents. Moreover, the abysmal patient 5-year survival rate has remained constant since several years, further emphasizing the importance of developing newer therapies that are efficacious against lung cancer. Smoking cessation and chemoprevention strategies are two major practical approaches that can reduce the large number of lung cancer related-mortalities. Successful studies conducted so far, have convincingly established the chemopreventive and anti-cancer efficacy of silibinin. The predominant role of silibinin in regulating pleiotropic mitogenic signaling cascades involved in controlling prime endpoints like cell proliferation, cell survival and cell cycle progression, has helped define its efficacy at different stages of cancer, as well exploit its potential activity against several epithelial cancers including skin, prostate, colon, and lung. The importance of its biological relevance lies in its properties which include its non-toxicity, acceptability, and safety for human consumption, warranting its use in Phase I/II clinical trials. Collectively, significant findings have highlighted the efficacious role of silibinin in a plethora of cancers including lung cancer. Specifically in lung cancer studies, silibinin was effective either alone or in combination with other agents (e.g. chemotherapeutic and epigenetic agents) in significantly inhibiting the growth of lung cancer cells. In vivo, its effects have been shown to be mediated through inhibition of proliferation, angiogenesis and epigenetic related events. Therefore, in support of these studies, the strong chemopreventive effects of silibinin and their associated mechanisms merit further investigations.
Acknowledgments
NCI grants R01 CA113876 and CA102514.
Footnotes
Conflict of Interest Disclosures: The authors declare that there are no conflicts to disclose.
References
- 1.Amercian Cancer Society. Cancer Facts and Figures. 2012 [Google Scholar]
- 2.Denlinger CE, Ikonomidis JS, Reed CE, Spinale FG. Epithelial to mesenchymal transition: the doorway to metastasis in human lung cancers. J Thorac Cardiovasc Surg. 2010;140:505–513. doi: 10.1016/j.jtcvs.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD. Classification of lung cancer. Semin Roentgenol. 2011;46:178–186. doi: 10.1053/j.ro.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH. Current standards of care in small-cell and non-small-cell lung cancer. Oncology. 2001;61 (Suppl 1):3–13. doi: 10.1159/000055386. [DOI] [PubMed] [Google Scholar]
- 5.Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75:56–63. [PubMed] [Google Scholar]
- 6.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 7.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 8.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina JR, Adjei AA, Jett JR. Advances in chemotherapy of non-small cell lung cancer. Chest. 2006;130:1211–1219. doi: 10.1378/chest.130.4.1211. [DOI] [PubMed] [Google Scholar]
- 10.Sorenson S, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in non-small cell lung cancer. Acta Oncol. 2001;40:327–339. doi: 10.1080/02841860151116402. [DOI] [PubMed] [Google Scholar]
- 11.D’Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, et al. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005;23:2926–2936. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Schiller JH, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. J Clin Invest. 2007;117:2740–2750. doi: 10.1172/JCI31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burris HA., 3rd Shortcomings of current therapies for non-small-cell lung cancer: unmet medical needs. Oncogene. 2009;28 (Suppl 1):S4–13. doi: 10.1038/onc.2009.196. [DOI] [PubMed] [Google Scholar]
- 14.Giaccone G. Twenty-five years of treating advanced NSCLC: what have we achieved? Ann Oncol. 2004;15(Suppl 4):iv81–83. doi: 10.1093/annonc/mdh908. [DOI] [PubMed] [Google Scholar]
- 15.Neal JW, Sequist LV. Exciting new targets in lung cancer therapy: ALK, IGF-1R, HDAC, and Hh. Curr Treat Options Oncol. 2010;11:36–44. doi: 10.1007/s11864-010-0120-6. [DOI] [PubMed] [Google Scholar]
- 16.Sangha R, Lara PN, Jr, Mack PC, Gandara DR. Beyond antiepidermal growth factor receptors and antiangiogenesis strategies for nonsmall cell lung cancer: exploring a new frontier. Curr Opin Oncol. 2009;21:116–123. doi: 10.1097/CCO.0b013e3283210489. [DOI] [PubMed] [Google Scholar]
- 17.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 18.Cohen V, Khuri FR. Progress in lung cancer chemoprevention. Cancer Control. 2003;10:315–324. doi: 10.1177/107327480301000406. [DOI] [PubMed] [Google Scholar]
- 19.Cohen V, Khuri FR. Chemoprevention of lung cancer. Curr Opin Pulm Med. 2004;10:279–283. doi: 10.1097/01.mcp.0000129754.97392.d5. [DOI] [PubMed] [Google Scholar]
- 20.Soria JC, Kim ES, Fayette J, Lantuejoul S, Deutsch E, et al. Chemoprevention of lung cancer. Lancet Oncol. 2003;4:659–669. doi: 10.1016/s1470-2045(03)01244-0. [DOI] [PubMed] [Google Scholar]
- 21.Sporn MB. Chemoprevention of cancer. Lancet. 1993;342:1211–1213. doi: 10.1016/0140-6736(93)92189-z. [DOI] [PubMed] [Google Scholar]
- 22.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533–544. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 24.van Zandwijk N, Hirsch FR. Chemoprevention of lung cancer: current status and future prospects. Lung Cancer. 2003;42 (Suppl 1):S71–79. doi: 10.1016/s0169-5002(03)00307-6. [DOI] [PubMed] [Google Scholar]
- 25.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharm Res. 2010;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 26.Omenn GS. Chemoprevention of lung cancers: lessons from CARET, the beta-carotene and retinol efficacy trial, and prospects for the future. Eur J Cancer Prev. 2007;16:184–191. doi: 10.1097/01.cej.0000215612.98132.18. [DOI] [PubMed] [Google Scholar]
- 27.Omenn GS. Human lung cancer chemoprevention strategies: Parker B. Francis lecture. Chest. 2004;125:123S–127S. doi: 10.1378/chest.125.5_suppl.123s. [DOI] [PubMed] [Google Scholar]
- 28.Winterhalder RC, Hirsch FR, Kotantoulas GK, Franklin WA, Bunn PA., Jr Chemoprevention of lung cancer--from biology to clinical reality. Ann Oncol. 2004;15:185–196. doi: 10.1093/annonc/mdh051. [DOI] [PubMed] [Google Scholar]
- 29.Cohen V, Khuri FR. Chemoprevention of lung cancer: current status and future prospects. Cancer Metastasis Rev. 2002;21:349–362. doi: 10.1023/a:1021223313546. [DOI] [PubMed] [Google Scholar]
- 30.Lam S, McWilliams A, LeRiche J, MacAulay C, Wattenberg L, et al. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526–1531. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 31.Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 32.Kim KW, Choi CH, Kim TH, Kwon CH, Woo JS, et al. Silibinin inhibits glioma cell proliferation via Ca2+/ROS/MAPK-dependent mechanism in vitro and glioma tumor growth in vivo. Neurochem Res. 2009;34:1479–1490. doi: 10.1007/s11064-009-9935-6. [DOI] [PubMed] [Google Scholar]
- 33.Keith RL. Chemoprevention of lung cancer. Proc Am Thorac Soc. 2009;6:187–193. doi: 10.1513/pats.200807-067LC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh RP, Agarwal R. Prostate cancer prevention by silibinin. Curr Cancer Drug Targets. 2004;4:1–11. doi: 10.2174/1568009043481605. [DOI] [PubMed] [Google Scholar]
- 36.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 39.Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (Silybum marianum) Integr Cancer Ther. 2007;6:104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 41.Rainone F. Milk thistle. Am Fam Physician. 2005;72:1285–1288. [PubMed] [Google Scholar]
- 42.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 43.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells. Mol Cancer Ther. 2008;7:1817–1826. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mateen S, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog. 2010;49:247–258. doi: 10.1002/mc.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramasamy K, Dwyer-Nield LD, Serkova NJ, Hasebroock KM, Tyagi A, et al. Silibinin prevents lung tumorigenesis in wild-type but not in iNOS−/− mice: potential of real-time micro-CT in lung cancer chemoprevention studies. Clin Cancer Res. 2011;17:753–761. doi: 10.1158/1078-0432.CCR-10-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 47.Tyagi A, Agarwal C, Dwyer-Nield LD, Singh RP, Malkinson AM, et al. Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog. 2011 doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- 48.Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23:2649–2655. [PubMed] [Google Scholar]
- 49.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, et al. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF, et al. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J Dent Res. 2006;85:220–225. doi: 10.1177/154405910608500303. [DOI] [PubMed] [Google Scholar]
- 52.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40:143–149. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- 53.Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, et al. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 54.Rho JK, Choi YJ, Jeon BS, Choi SJ, Cheon GJ, et al. Combined treatment with silibinin and epidermal growth factor receptor tyrosine kinase inhibitors overcomes drug resistance caused by T790M mutation. Mol Cancer Ther. 2010;9:3233–3243. doi: 10.1158/1535-7163.MCT-10-0625. [DOI] [PubMed] [Google Scholar]
- 55.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila) 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dagne A, Melkamu T, Schutten MM, Qian X, Upadhyaya P, et al. Enhanced inhibition of lung adenocarcinoma by combinatorial treatment with indole-3–carbinol and silibinin in A/J mice. Carcinogenesis. 2011;32:561–567. doi: 10.1093/carcin/bgr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Y, Wang Y, Tan Q, Lubet RA, You M. Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2005;7:1053–1057. doi: 10.1593/neo.05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- 59.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Risch A, Plass C. Lung cancer epigenetics and genetics. Int J Cancer. 2008;123:1–7. doi: 10.1002/ijc.23605. [DOI] [PubMed] [Google Scholar]
- 61.Mateen S, Raina K, Agarwal C, Agarwal R. Inhibition of Epigenetic Chromatin-Modification Enzymes: Histone Deacetylases and DNA Methyltransferases by Silibinin in human NSCLC H1299 cells. 101 st Annual Meeting of AACR; Washington D.C, USA. April 2010; p. abstr. [Google Scholar]
- 62.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Bots M, Johnstone RW. Rational combinations using HDAC inhibitors. Clin Cancer Res. 2009;15:3970–3977. doi: 10.1158/1078-0432.CCR-08-2786. [DOI] [PubMed] [Google Scholar]
- 64.Song SH, Han SW, Bang YJ. Epigenetic-based therapies in cancer: progress to date. Drugs. 2011;71:2391–2403. doi: 10.2165/11596690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 65.Mateen S, Raina K, Agarwal C, Chan D, Agarwal R. Silibinin synergizes with Histone Deacetylase (HDAC) inhibitors in upregulating E-cadherin expression in non-small lung cancer (NSCLC) cells. 103rd Annual Meeting of AACR; Chicago, USA. April 2012; p. abstr. [Google Scholar]
- 66.Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 67.Richardson F, Young GD, Sennello R, Wolf J, Argast GM, et al. The evaluation of E-Cadherin and vimentin as biomarkers of clinical outcomes among patients with non-small cell lung cancer treated with erlotinib as second- or third-line therapy. Anticancer Res. 2012;32:537–552. [PubMed] [Google Scholar]